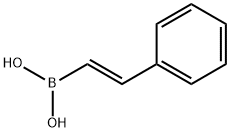
E-PHENYLETHENYLBORONIC ACID synthesis
- Product Name:E-PHENYLETHENYLBORONIC ACID
- CAS Number:6783-05-7
- Molecular formula:C8H9BO2
- Molecular Weight:147.97
![[(Z)-2-bromoethenyl]benzene](/CAS/GIF/588-72-7.gif)
588-72-7

6783-05-7
The general procedure for the synthesis of trans-BETA-styrene boronic acid from (E)-(2-bromovinyl)benzene is as follows: in a 50 mL round-bottomed flask (equipped with a side arm, condenser, and stirring bar) triphenylphosphine (0.131 g, 0.5 mmol, 20 mol%), p-iodoanisole (0.585 g, 2.5 mmol), and triethylamine (1.78 mL. 12.5 mmol). The reaction system was degassed three times by alternating vacuum and argon filling. Palladium dichloride (0.023 g, 0.13 mmol, 5 mol%) was added under positive argon atmosphere. After stirring for 15 minutes at room temperature, diisopropylaminoborane (5 mL, 1 M THF solution, 5 mmol) was added and the reaction mixture was again degassed three times by alternating vacuum and argon filling. The reaction mixture was heated to reflux and kept at reflux for 12 hours. Upon completion of the reaction, the reaction solution was cooled to 0 °C, and 6 mL of methanol was slowly added (note: this process is an exothermic reaction accompanied by hydrogen release). Stirring was continued for 15 minutes and then concentrated under reduced pressure to remove all solvent to give a black solid. The solid was dissolved in 3M sodium hydroxide solution (8 mL) and subsequently washed with hexane (3 x 10 mL). The aqueous phase was cooled to 0°C (ice bath) and acidified with concentrated hydrochloric acid to pH ≤ 1, at which point the boric acid precipitated as a white solid. The aqueous phase was extracted with ether (3 x 10 mL), the organic phases were combined, dried with magnesium sulfate and filtered. Finally, the solvent was removed by concentration under reduced pressure to obtain the target product, trans-BETA-styrene boronic acid, as a white solid.
![[(Z)-2-bromoethenyl]benzene](/CAS/GIF/588-72-7.gif)
588-72-7
4 suppliers
inquiry

6783-05-7
116 suppliers
$24.00/1g
Yield:6783-05-7 51%
Reaction Conditions:
Stage #1: [(E)-2-bromoethenyl]benzenewith tris(dibenzylideneacetone)dipalladium(0) chloroform complex;diisopropopylaminoborane;triethylamine;triphenylphosphine in tetrahydrofuran at 65; for 12 h;Inert atmosphere;Alcaraz-Vaultier borylation;
Stage #2: with methanol in tetrahydrofuran at 0;Inert atmosphere;Further stages;
Steps:
4.2. General procedure for the synthesis of boronic acids from the reaction of aryl iodides, bromides, or triflates with BH2N(iPr)2 in the presence of a palladium catalyst
General procedure: Triphenylphosphene (0.131 g, 0.5 mmol, 20 mol %), p-iodoanisol (0.585 g, 2.5 mmol), and triethylamine (1.78 mL, 12.5 mmol) were added to a 50 mL round-bottomed flask equipped with a sidearm, condenser, and stir bar. This solution was then degassed by alternating vacuum and argon three times. Palladium dichloride (0.023 g, 0.13 mmol, 5 mol %) was then added under positive argon pressure. After stirring at room temperature for 15 min, diisopropylaminoborane (5 mL, 1 M solution in THF, 5 mmol) was added and the reaction mixture was degassed again by alternating vacuum and argon three times. The reaction solution was then heated to reflux. After 12 h of reflux the reaction was cooled to 0 °C and 6 mL of methanol was added through the condenser slowly (Caution: exothermic reaction with evolution of hydrogen). After 15 min of stirring all the solvent was removed under reduced pressure to yield a black solid. This solid was dissolved with sodium hydroxide (3 M, 8 mL) and subsequently washed with hexanes (3×10 mL). The aqueous layer was then cooled to 0 °C (ice bath) and acidified to pH ≤1 with concentrated HCl, with the boronic acid usually precipitating out as a white solid. The aqueous fraction was then extracted with diethyl ether (3×10 mL). The organic fractions were combined, dried with magnesium sulfate and filtered. The solvent was then removed under reduced pressure yielding a white solid.
References:
Haddenham, Dustin;Bailey, Christopher L.;Vu, Chau;Nepomuceno, Gabby;Eagon, Scott;Pasumansky, Lubov;Singaram, Bakthan [Tetrahedron,2011,vol. 67,# 3,p. 576 - 583] Location in patent:experimental part
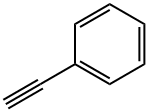
536-74-3
317 suppliers
$10.00/1g

6783-05-7
116 suppliers
$24.00/1g
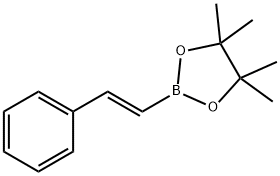
83947-56-2
112 suppliers
$19.00/250mg

6783-05-7
116 suppliers
$24.00/1g
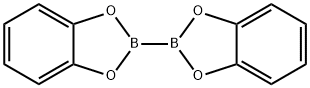
13826-27-2
142 suppliers
$5.00/250mg

536-74-3
317 suppliers
$10.00/1g

6783-05-7
116 suppliers
$24.00/1g
