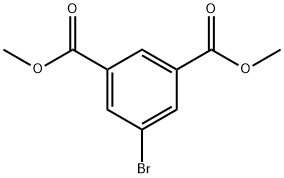
DIMETHYL 5-BROMOISOPHTHALATE synthesis
- Product Name:DIMETHYL 5-BROMOISOPHTHALATE
- CAS Number:51760-21-5
- Molecular formula:C10H9BrO4
- Molecular Weight:273.08
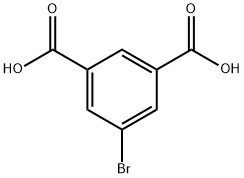
23351-91-9

51760-21-5
[Preparation of dimethyl 5-bromoisophthalate from 5-bromoisophthalic acid] To a 500 mL three-necked flask equipped with a mechanical stirrer and a Dimroth condenser, 110 g (0.45 mol) of 5-bromoisophthalic acid, 500 mL of anhydrous methanol, and 10 g of concentrated sulfuric acid were added sequentially. The reaction mixture was heated under reflux conditions with stirring for 6 hours. Upon completion of the reaction, the mixture was left to cool to room temperature, followed by slow dropwise addition to 1 L of distilled water. The mixture was centrifuged with 5 wt% aqueous sodium bicarbonate solution to pH 7-8. The precipitated white precipitate was collected by filtration and washed with 2 L of distilled water in two portions. The resulting white solid was placed in a vacuum drying oven and dried under reduced pressure at 50 °C for 48 h to give 109 g (0.4 mol) of dimethyl 5-bromoisophthalate in 89% yield.

23351-91-9
163 suppliers
$6.00/1g

51760-21-5
193 suppliers
$6.00/1g
Yield:51760-21-5 89%
Reaction Conditions:
with sulfuric acid;sodium hydrogencarbonate in methanol
Steps:
3 [Preparation of dimethyl 5-bromoisophthalate from 5-bromoisophthalic acid]
[Preparation of dimethyl 5-bromoisophthalate from 5-bromoisophthalic acid] Into a 500 ml flask equipped with a stirrer and a Dimroth condenser, 110 g of 5-bromoisophthalic acid obtained above, 500 ml of methanol and 10 g of a concentrated sulfuric acid were placed and the resultant mixture was heated under the refluxing condition for 6 hours. After the reaction mixture was cooled by being left standing, the cooled mixture was added dropwise to 1 liter of distilled water and the obtained mixture was neutralized with a 5% by weight aqueous solution of sodium hydrogencarbonate. The formed precipitates were separated by filtration and washed twice with 2 liters of distilled water. The obtained white solid substance was dried at 50?C under a reduced pressure for 2 days and 109 g (0.4 moles) of dimethyl 5-bromoisophthalate was obtained (the yield: 89%).
References:
SUMITOMO BAKELITE CO., LTD. EP1346975, 2003, A1
99-27-4
281 suppliers
$6.00/5g

51760-21-5
193 suppliers
$6.00/1g

23351-91-9
163 suppliers
$6.00/1g

74-88-4
356 suppliers
$15.00/10g

51760-21-5
193 suppliers
$6.00/1g

67-56-1
782 suppliers
$7.29/5ml-f

23351-91-9
163 suppliers
$6.00/1g

51760-21-5
193 suppliers
$6.00/1g

67-56-1
782 suppliers
$7.29/5ml-f
121-91-5
607 suppliers
$6.00/10g
1459-93-4
336 suppliers
$11.00/100g

51760-21-5
193 suppliers
$6.00/1g