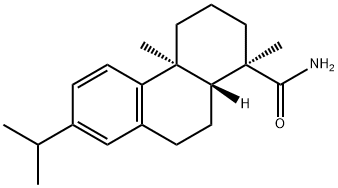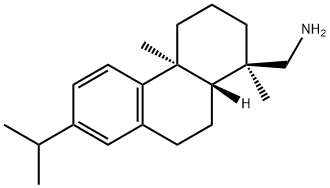
DEHYDROABIETYLAMINE synthesis
- Product Name:DEHYDROABIETYLAMINE
- CAS Number:1446-61-3
- Molecular formula:C20H31N
- Molecular Weight:285.47
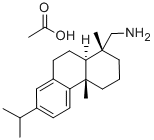
2026-24-6

1446-61-3
The general procedure for the synthesis of dehydroabietic amine from dehydroabietic amine acetate was as follows: crude 60% (+)-dehydroabietic amine (42.0 g) was dissolved in toluene (70.0 cm3) and a toluene (30.0 cm3) solution of acetic acid (9.65 g) was slowly added. The mixture was crystallized in a refrigerator. The product was collected by filtration and washed with hexane (30.0 cm3). The (+)-dehydrofiramine ethanol compound was recrystallized from methanol. (+)-Dehydrofiramine acetate (21.0 g) was dissolved in hot water and 10% NaOH aqueous solution (28.0 cm3) was added. The (+)-dehydrofiramine was extracted with diethyl ether (50.0 cm3) and the organic phase was washed with water to neutrality and subsequently dried over anhydrous sodium sulfate. The solvent was evaporated and the (+)-dehydrofiramine obtained was dried in vacuum to give a white solid; yield 37.0 g, 88.2%; melting point 44.28 °C (literature value 44-45 °C [16]). [α]22D +44.3480 (c, 10.0 mg/cm3, CHCl?).1H NMR (500 MHz, CDCl?) δ 0.89 (s, 3H, CH?), 1.22 (s, 3H, CH?), 1.22 (d, J=7.0 Hz, 6H, 2 × CH?), 1.33 (m, 2H, CH?), 1.39 ( m, 1H, CHH), 1.52 (dd, J=11.8,3.3Hz, 1H, CH), 1.69 (m, 2H, CH?), 1.74 (m, 2H, CH?), 2.30 (dt, J=13.1,1.7Hz, 1H, CHH), 2.40 (d, J=13.5Hz, 1H, CHH), 2.61 (d, J= 13.5 Hz, 1H, CHH), 2.82 (sep, J=7.0 Hz, CH), 2.88 (m, 2H, CH?), 6.89 (d, J=1.9 Hz, 1H, CHAr), 7.00 (dd, J=8.1,1.9 Hz, 1H, CHAr), 7.18 (d, J=8.1 Hz, 1H, CHAr). 13C NMR (500 MHz, CDCl?) δ 18.78 (CH?), 18.90 (CH?), 18.90 (CH?), 24.11 (CH?), 24.13 (CH?), 25.37 (CH?), 30.31 (CH?), 33.58 (CH), 35.36 (CH?), 37.36 (C). 37.53 (C), 38.70 (CH?), 45.00 (CH), 53.99 (CH?), 123.96 (CHAr), 124.38 (CHAr), 126.94 (CHAr), 134.84 (CAr), 145.67 (CAr), 147.63 (CAr).HRMS-ESI m/z 286.2540; C??H??N [M+H]? Calculated value 286.2529.

2026-24-6
11 suppliers
inquiry

1446-61-3
224 suppliers
$9.00/5g