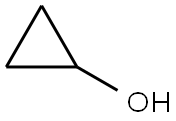
cyclopropanol synthesis
- Product Name:cyclopropanol
- CAS Number:16545-68-9
- Molecular formula:C3H6O
- Molecular Weight:58.08

411235-57-9

16545-68-9
Step 1: Synthesis of cyclopropyl-1-ol Cyclopropylboronic acid (10 g, 0.116 mol) and aqueous sodium hydroxide (8.37 g, 0.209 mol, dissolved in 100 mL of water) were added to a 1 L reaction flask. Under the cooling of an ice bath, 34% hydrogen peroxide (80 mL) was slowly added dropwise, keeping the reaction temperature not higher than 5 °C. After the dropwise addition, the reaction mixture was continued to be stirred at 5°C for 1 hour. Upon completion of the reaction, saturated aqueous sodium thiosulfate solution was added slowly and dropwise to quench the reaction until the potassium iodide-starch test paper no longer showed color. The reaction solution was extracted with ether three times, the organic phases were combined, washed with saturated brine, dried with anhydrous desiccant, filtered and concentrated under reduced pressure at 0 °C to give cyclopropan-1-ol (colorless oil, 4 g, 60% yield). The product did not need further purification and could be used directly in the subsequent reaction. (MS detection: [M+1] not observed)

411235-57-9
433 suppliers
$6.00/1g

16545-68-9
116 suppliers
$70.00/100 mg
Yield:16545-68-9 60%
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in water at 5; for 1 h;
Steps:
1 Step 1: cyclopropanol
Step 1: cyclopropanol (0104) (0105) Cyclopropylboronic acid (10g, 0.116mol), sodium hydroxide aqueous solution (8.37g, 0.209mol, added to 100ml water) were added into a 1L reaction flask, and hydrogen peroxide (34%, 80mL) was slowly dropped thereinto under ice bath and the temperature was kept not higher than 5°Cduring the process of dropping. After adding, the mixture was stirred at 5°C for 1 hour. After completion of the reaction, a saturated sodium thiosulfate aqueous solution was slowly dropped to terminate the reaction until the potassium iodide-starch test paper does not change color. The reaction solution was extracted with diethyl ether for three times and the combined organic phase was washed with saturated brine, dried, filtered and concentrated at 0°C to obtain the title compound (colorless oil, 4g, 60%), which may be used directly for the subsequent reaction. (MS: [M+1] none)
References:
Beijing Pearl Biotechnology Limited Liability Company;DONG, Jiaqiang;ZHONG, Boyu;YUAN, Hongbin;SHI, Quan;CHU, Shaosong;ZHANG, Deyi;ZHANG, Ruihao EP3150592, 2017, A1 Location in patent:Paragraph 0103; 0104; 0105
75-19-4
42 suppliers
$75.00/5 g

16545-68-9
116 suppliers
$70.00/100 mg
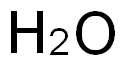
7732-18-5
498 suppliers
$12.69/100ml

411235-57-9
433 suppliers
$6.00/1g

16545-68-9
116 suppliers
$70.00/100 mg
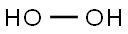
7722-84-1
0 suppliers
$23.30/*2x50ml

411235-57-9
433 suppliers
$6.00/1g

16545-68-9
116 suppliers
$70.00/100 mg
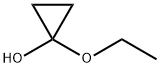
13837-45-1
40 suppliers
inquiry

16545-68-9
116 suppliers
$70.00/100 mg