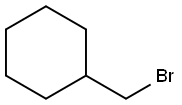
Cyclohexylmethyl bromide synthesis
- Product Name:Cyclohexylmethyl bromide
- CAS Number:2550-36-9
- Molecular formula:C7H13Br
- Molecular Weight:177.08
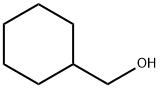
100-49-2

2550-36-9
The general procedure for the synthesis of bromomethylcyclohexane from cyclohexanemethanol is as follows: 1.4 kg of cyclohexanemethanol (colorless liquid), 8.4 L of toluene, and 969.8 g of pyridine were added to a 20 L four-neck flask. The reaction system was cooled to 0 to 10 °C, and then a mixture of 1.66 kg of phosphorus tribromide with 7 L of toluene was slowly added with controlled titration rate to keep the reaction temperature below 5 °C. The dropwise addition process was completed in about 1 hour. Subsequently, the reaction system was warmed to room temperature and the reaction was continued with stirring for 10 hours. Upon completion of the reaction, the system was cooled to below 20 °C and about 2.5 L of 5% sodium hydroxide solution was slowly added dropwise, followed by the addition of 1.85 kg of solid sodium hydroxide to form a mixture. Liquid-liquid separation was carried out and the aqueous phase was extracted twice with 4 L of toluene. The organic phases were combined and washed with saturated brine, and the washed organic phase was dried with anhydrous sodium sulfate. The dried organic phase was concentrated to give 1.73 kg of crude product. The crude product was purified by distillation under reduced pressure to give 722.8 g of bromomethylcyclohexane with 97.5% purity in 33.3% yield. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 3.82 (m, 1H), 1.79-1.53 (m, 6H), 1.13-0.90 (m, 6H).
3725-11-9
12 suppliers
inquiry

2550-36-9
279 suppliers
$5.00/1g
Yield:2550-36-9 90.3%
Reaction Conditions:
with 15-crown-5;sodium bromide at 20 - 80;Temperature;Reagent/catalyst;
Steps:
3 Example 3
200 g of cyclohexylmethanol and 222.6 g of sodium carbonate were added to 1 L of toluene, stirred and cooled to 10-20 C.At this temperature, 515 g of p-toluenesulfonyl chloride (dissolved in 2L of toluene) was added dropwise over 2.5 hours. After the dropwise addition, the amount was increased to 30.Esterification at ~35°C until the cyclohexane methanol GC content is 3% and filtration to obtain the organic phase intermediate filtrate, intermediate the organic phaseThe body filtrate was transferred to a 5L four-necked flask equipped with a magnetic stirrer, and 344 g of sodium bromide was added thereto while stirring at 20-35°C.15-crown-5catalyst 10g, after the completion of the addition, the temperature was raised to 70-80°C, and the reaction was incubated until the organic phase intermediate GC content was 3.6%.At 40°C, the organic phase filtrate is filtered and recovered. Distillation of toluene is performed and distillation is performed to obtain a pale yellow transparent product. The pale yellow transparent product is dried.After the product (bromomethyl)cyclohexane140.2 g, GC content 98%, yield 90.3%.
References:
Changzhou Woteng Chemical Technology Co., Ltd.;Sun Bo;Liu Zuhe;Yao Yuan;Xie Rong CN107652162, 2018, A Location in patent:Paragraph 0010-0013

100-49-2
331 suppliers
$6.00/10g

2550-36-9
279 suppliers
$5.00/1g
931-50-0
113 suppliers
$45.00/1g

2550-36-9
279 suppliers
$5.00/1g
108-85-0
343 suppliers
$5.00/10g

2550-36-9
279 suppliers
$5.00/1g
2043-61-0
340 suppliers
$6.00/5g

2550-36-9
279 suppliers
$5.00/1g