
cycloheptanecarboxamide synthesis
- Product Name:cycloheptanecarboxamide
- CAS Number:1459-39-8
- Molecular formula:C8H15NO
- Molecular Weight:141.21
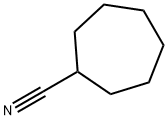
32730-85-1

1459-39-8
General procedure for the synthesis of cycloheptanamide from cycloheptanecarbonitrile: To a solution of nitromethane (1 mL) containing cycloheptanecarbonitrile (1.00 g, 8.12 mmol) was added sequentially, at room temperature, water (1 mL), DBU (2.47 g, 16.2 mmol), copper(I) iodide (309 mg, 1.62 mmol), and caesium carbonate(I) (1.32 g, 4.06 mmol). The reaction mixture was heated to 90°C and maintained for 8 hours. After completion of the reaction, the mixture was poured into water (50 mL) and the organic layer was separated. The aqueous layer was extracted with chloroform (CHCl3), the organic layers were combined and dried with anhydrous magnesium sulfate (MgSO4). The solvent was removed by concentration under reduced pressure and the resulting brown solid was washed with hexane to give the final cycloheptanamide (499 mg, 44% yield) with a melting point of 192-194 °C.

32730-85-1
40 suppliers
$164.00/5g

1459-39-8
37 suppliers
$49.00/100mg
Yield:1459-39-8 44%
Reaction Conditions:
with copper(l) iodide;caesium carbonate;1,8-diazabicyclo[5.4.0]undec-7-ene in nitromethane;water at 20 - 100; for 3 h;
Steps:
Synthesis of Cycloheptanecarboxamide (5m).S14
To a nitromethane (1 mL) solution of cycloheptanecarbonitrile (4m) (1.00 g, 8.12 mmol) were addedH2O (1 mL), DBU (2.47 g, 16.2 mmol), copper (I) iodide (309 mg, 1.62 mmol), cesium (I) carbonate(1.32 g, 4.06 mmol) at room temperature. The reaction mixture was heated at 90 °C for 8 h and thenpoured into water (50 mL). The organic layer was separated and the aqueous layer was extractedwith CHCl3. The combined organic layer was dried over MgSO4. The solvent was removed underreduced pressure. The formed brown powders were washed with n-hexane to give the titledcompound (499 mg, 44%).mp 192-194 °C,
References:
Kuwabara, Jun;Sawada, Yoshiharu;Yoshimatsu, Mitsuhiro [Synlett,2018,vol. 29,# 15,p. 2061 - 2065] Location in patent:supporting information
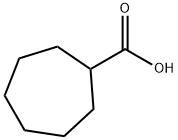
1460-16-8
142 suppliers
$25.00/1g

1459-39-8
37 suppliers
$49.00/100mg
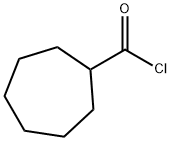
6557-86-4
28 suppliers
$134.31/250mgs:

1459-39-8
37 suppliers
$49.00/100mg
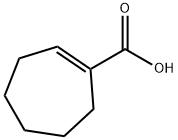
4321-25-9
5 suppliers
inquiry

1459-39-8
37 suppliers
$49.00/100mg
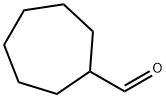
4277-29-6
42 suppliers
$45.00/25mg

1459-39-8
37 suppliers
$49.00/100mg