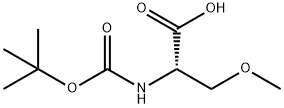
BOC-SER(ME)-OH synthesis
- Product Name:BOC-SER(ME)-OH
- CAS Number:51293-47-1
- Molecular formula:C9H17NO5
- Molecular Weight:219.24
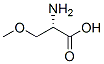
4219-94-7

24424-99-5

51293-47-1
General procedure for the synthesis of N-Boc-O-methyl-L-serine from O-methyl DL-serine and di-tert-butyl dicarbonate: 35 mL of aqueous 1 N sodium hydroxide solution and 35 mL of tetrahydrofuran (THF) were added to 2.0 g (17 mmol) of O-methyl DL-serine. With stirring at 0 °C, a solution of di-tert-butyl dicarbonate (4.03 g, 18.5 mmol) in THF (15 mL) was added slowly. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the reaction mixture was concentrated by evaporation under reduced pressure. The aqueous phase was acidified to pH 4-5 with 10% aqueous citric acid and then extracted with ethyl acetate. The organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give 3.6 g of N-Boc-O-methyl-L-serine (98% yield). Mass spectrum (ESI) m/z: 220.1 (M + H)+.

75-18-3
408 suppliers
$18.10/8208330005
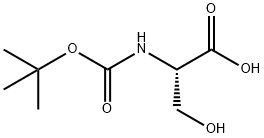
3262-72-4
404 suppliers
$10.00/1g

51293-47-1
205 suppliers
$8.00/250mg
Yield:51293-47-1 240.3 kg
Reaction Conditions:
with sodium hydroxide at 10; for 44 h;Large scale;
Steps:
2 Example 2 Synthesis of (S)-2-((tert-butoxycarbonyl)amino)-3-methoxypropanoic acid
Example 2 Synthesis of (S)-2-((tert-butoxycarbonyl)amino)-3-methoxypropanoic acid (0033) (0034) 45% 4 NaOH (746.3 kg; 8.40 kmoles) and DMS (1053.8 kg; 8.36 kmoles) were added to the reaction mixture below 10° C. over 38 h and for an additional 6 h to complete the reaction. 20% 9 NH4OH (20.0 kg) was added to quench the reaction in the reaction mixture. The pH value was adjusted by 10 citric acid monohydrate (55.8 kg) and 32% 11 HCl (180 kg) to around 3. The 12 product was extracted by toluene (1165 L) twice, and the organic layer was washed by 1% NaOH (40 L) and H2O (39 L×2). The organic layer was concentrated, and stripped by IPA as oil residue (240.3 kg)
References:
SCI Pharmtech, Inc.;Chen, Bo-Fong;Su, Yen-Chi;Li, Yen-Wei;Li, Feng-Hsu;Huang, Chen-Wei US9718765, 2017, B1 Location in patent:Page/Page column 5; 6

3262-72-4
404 suppliers
$10.00/1g

74-88-4
358 suppliers
$15.00/10g

51293-47-1
205 suppliers
$8.00/250mg
![L-?Serine, N-?[(1,?1-?dimethylethoxy)?carbonyl]?-?O-?methyl-?, phenylmethyl ester](/CAS/20210111/GIF/183793-47-7.gif)
183793-47-7
2 suppliers
inquiry

51293-47-1
205 suppliers
$8.00/250mg
![L-Serine, N-[(1,1-diMethylethoxy)carbonyl]-O-Methyl-, coMpd. with N-cyclohexylcyclohexanaMine (1:1)](/CAS/GIF/69912-63-6.gif)
69912-63-6
41 suppliers
inquiry

51293-47-1
205 suppliers
$8.00/250mg
![L-Serine, N-[(1,1-diMethylethoxy)carbonyl]-O-Methyl-, Methyl ester](/CAS/GIF/134167-07-0.gif)
134167-07-0
33 suppliers
$58.00/100mg

51293-47-1
205 suppliers
$8.00/250mg