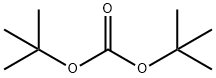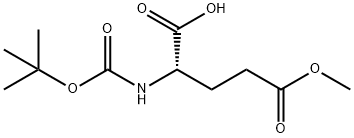
BOC-GLU(OME)-OH synthesis
- Product Name:BOC-GLU(OME)-OH
- CAS Number:45214-91-3
- Molecular formula:C11H19NO6
- Molecular Weight:261.27

1499-55-4

24424-99-5

45214-91-3
In a round-bottomed flask, triethylamine (188 g, 1.86 mol, 1.0 eq.) was slowly added dropwise to a stirred mixed solution containing di-tert-butyl dicarbonate (162 g, 0.744 mol, 1.2 eq.) and L-glutamic acid-5-methyl ester (100 g, 0.62 mol, 1.0 eq.) in a solvent consisting of a mixture of water (500 mL) and 1,4-dioxane (500 mL) in a solvent mixture of water (500 mL) and 1,4-dioxane (500 mL). The reaction mixture was stirred continuously for 18 hours at room temperature. Upon completion of the reaction, the reaction solution was extracted with methyl tert-butyl ether (MTBE, 500 mL x 2). The aqueous phase was cooled in an ice bath and the pH was carefully adjusted to 3 by slowly adding 10% citric acid solution.Subsequently, the carbamate product in the aqueous phase was extracted with ethyl acetate (500 mL × 2). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford (S)-2-((tert-butoxycarbonyl)amino)-5-methoxy-5-oxopentanoic acid (Compound A4-2) as a clear viscous oil (180 g, 100% yield). Mass spectrometry (MS-ESI) analysis showed [M + 1]+ m/z 262.1.

1499-55-4
302 suppliers
$6.00/5g

24424-99-5
858 suppliers
$13.50/25G

45214-91-3
198 suppliers
$10.00/5g
Yield:45214-91-3 100%
Reaction Conditions:
with triethylamine in 1,4-dioxane;water at 20; for 18 h;
Steps:
4.1 Step 1
In a round bottom flask, triethylamine (188g, 1.86mol, l .Oeq) was added dropwise to a stirred solution of di-tert-butyl dicarbonate (162g, 0.744mol, 1.2eq) and compound A4-1 (lOOg, 0.62mol, l .Oeq) in water (500mL) and 1,4-dioxane (500mL). After stirring for 18hrs at room temperature, the solution was extracted with MTBE (500mL*2) and the aqueous phase was cooled on ice and carefully acidified to pH 3 by slow addition of 10% citric acid solution. The urethane was then extracted twice with ethyl acetate, and the combined extracts was washed with brine, dried over anhydrous sodium sulfate, and concentrated to give compound A4-2 as clear viscous oil (180g, yield 100%). MS-ESI:[M+1]+: 262.1
References:
LIANG, Congxin WO2018/67422, 2018, A1 Location in patent:Page/Page column 47
132245-78-4
15 suppliers
inquiry

45214-91-3
198 suppliers
$10.00/5g

24424-99-5
858 suppliers
$13.50/25G

3077-51-8
8 suppliers
inquiry

45214-91-3
198 suppliers
$10.00/5g

1499-55-4
302 suppliers
$6.00/5g

45214-91-3
198 suppliers
$10.00/5g