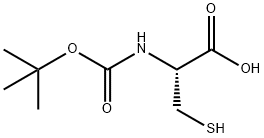
BOC-CYS-OH synthesis
- Product Name:BOC-CYS-OH
- CAS Number:20887-95-0
- Molecular formula:C8H15NO4S
- Molecular Weight:221.27

10389-65-8

20887-95-0
General procedure for the synthesis of (R)-2-((tert-butoxycarbonyl)amino)-3-mercaptopropionic acid from N,N'-bis(Boc)-L-cystine: N,N'-bis(Boc)-L-cystine (2.51 mmol) was dissolved in a solvent mixture of tetrahydrofuran (29.2 mL) and water (0.8 mL). Tributylphosphine (2.76 mmol) was added to the solution under ice bath cooling conditions. The reaction mixture was gradually warmed up to room temperature and stirred continuously at this temperature for 1 hour. Upon completion of the reaction, ethyl acetate (20 mL) and 10% aqueous citric acid (10 mL) were added to the reaction mixture for extraction and separation. The organic phase was collected and washed with saturated aqueous saline solution (20 mL). Subsequently, the organic phase was concentrated and the resulting residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate mixed solvent) to afford the target compound 22 as an oily product. Yield: 99%. ESI MS m/z 220.1 [M-H]-; 1H NMR (400 MHz, CDCl3) δ: 5.47 (1H, brs), 4.65 (1H, brs), 3.09-2.97 (2H, m), 1.48 (9H, s).
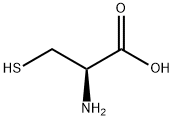
52-90-4
975 suppliers
$6.00/10g

24424-99-5
867 suppliers
$13.50/25G

20887-95-0
168 suppliers
$27.00/250mg
Yield:20887-95-0 97%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20;Cooling with ice;
Steps:
1.1 Step 1: Preparation of N-Boc-L-cysteine (1)
Add L-cysteine (6.0 g, 50 mmol), NaOH (1.2 g, 50 mmol),Dioxane (30 mL) and water (50 mL), (Boc) 2O (17.0 g, 70 mmol) were added under an ice bath, and stirring was continued at room temperature overnight. Concentrated under reduced pressure to remove dioxane,The aqueous layer was extracted with ethyl acetate to remove unreacted Boc anhydride.The aqueous layer was adjusted to pH 1-2 with 1 mol / L hydrochloric acid, and extracted twice with ethyl acetate.The organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulfate,Concentrated under reduced pressure to obtain 2.15 g (1) as a colorless syrup, with a yield of 97%.Directly used for the next reaction
References:
Fudan University;Chu Yong;Gao Yang;Ye Deyong CN110407770, 2019, A Location in patent:Paragraph 0036-0040

10389-65-8
140 suppliers
$14.00/5G

20887-95-0
168 suppliers
$27.00/250mg
5068-28-0
236 suppliers
$9.00/10g

20887-95-0
168 suppliers
$27.00/250mg
19746-37-3
222 suppliers
$6.00/1g

20887-95-0
168 suppliers
$27.00/250mg
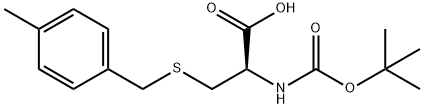
61925-77-7
166 suppliers
$9.00/1g

20887-95-0
168 suppliers
$27.00/250mg