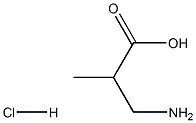
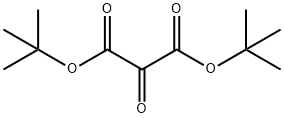
Boc-Aib-OH synthesis
- Product Name:Boc-Aib-OH
- CAS Number:30992-29-1
- Molecular formula:C9H17NO4
- Molecular Weight:203.24

24424-99-5

62-57-7

30992-29-1
The general procedure for the synthesis of 2-((tert-butoxycarbonyl)amino)-2-methylpropionic acid from di-tert-butyl dicarbonate and 2-aminoisobutyric acid is as follows: Preparation of Example 26: 2-(tert-butoxycarbonylamino)-2-methylpropionic acid 200 mg (1.94 mmol) of 2-amino-2-methylpropionic acid was dissolved in a solvent mixture of 8.0 mL of 1.0 N NaOH solution and 8.0 mL of 1,4-dioxane, followed by the addition of 846 mg (3.88 mmol) of di-tert-butyl dicarbonate (Boc2O). The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, 1,4-dioxane was removed by distillation under reduced pressure. The residue was adjusted to pH 3 with 1N HCl solution and then extracted with 320 mL of ethyl acetate. The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 315 mg of the white solid product 2-(tert-butoxycarbonylamino)-2-methylpropanoic acid in 80% yield. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 5.07 (brs, 1H), 1.54 (s, 6H), 1.45 (s, 9H).

24424-99-5
866 suppliers
$13.50/25G

62-57-7
452 suppliers
$10.64/25G

30992-29-1
287 suppliers
$6.00/5g
Yield:30992-29-1 90%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20; for 16 h;Cooling with ice;
Steps:
2-(1-(((tert-butoxycarbonyl) amino) methyl) cyclohexyl) aceticacid (3a)
General procedure: To a stirred solution of 2-(1-(aminomethyl) cyclohexyl)acetic acid 2a (2g, 11.68 mmol, 1equiv.) in 1, 4-dioxane (20 mL) atice temperature, aq. NaOH (560.57mg, 14.02 mmol, 1.2 equiv,dissolved in 10 mL water) was added followed by addition of ditert-butyl dicarbonate (5.10 gm, 23.36mmol, 2 equiv.) in 1,4-dioxane (5mL). The reaction mixture was stirred at room temperature for 16 h. The resulting suspension was concentrated in vacuo and the residuewas dissolved in water (10-15mL) and washed by ethyl acetate (15mL x 2). The resultingaqueous layer was acidified using dilute KHSO4 solution, followed by extraction withethyl acetate (30 mL x 3), dried (Na2SO4) and concentrated in vacuo to afford 3a as awhite solid, which was carried forward for the next reaction. Yield: 3.04g, 95%.
References:
Dangi, Abha;Marelli, Udaya Kiran;Meena, Chhuttan L.;Reichart, Florian;Sanjayan, Gangadhar J.;Singh, Dharmendra;Zahler, Stefan;Weinmüller, Michael [Bioorganic and medicinal chemistry letters,2020] Location in patent:supporting information

84758-55-4
42 suppliers
$39.00/100mg

30992-29-1
287 suppliers
$6.00/5g
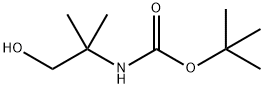
102520-97-8
132 suppliers
$7.00/1g

30992-29-1
287 suppliers
$6.00/5g