
Bisphenoxyethanolfluorene synthesis
- Product Name:Bisphenoxyethanolfluorene
- CAS Number:117344-32-8
- Molecular formula:C29H26O4
- Molecular Weight:438.51
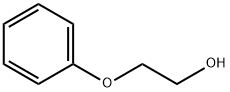
122-99-6
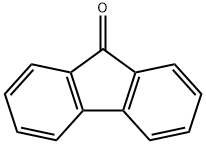
486-25-9

117344-32-8
The general procedure of Example 12 was as follows:(a) In a glass reactor equipped with a stirrer, a nitrogen introduction tube, a thermometer and a T-tube, 80.0 g (0.444 mol) of 9-fluorenone, 613.5 g (4.44 mol) of 2-phenoxyethanol and 2.0 g of tungstophosphoric acid catalyst [(H3PW12O40).nH2O] were added. (b) The mixture was reacted at 130°C for 4 hours under reduced pressure of 2.0 x 1.03 Pa. Upon completion of the reaction, the amount of polymers in the reaction mixture was 3.9%. To the reaction mixture was added 800.0 g of toluene, neutralized with aqueous sodium hydroxide and washed with water to separate the organic phase. Toluene and excess 2-phenoxyethanol were removed from the organic phase by concentration under reduced pressure. To the concentrate was added 560.0 g of toluene, and the mixture was heated and stirred at 80 °C for 1 hour, then cooled to 70 °C. 0.4 g of 9,9-bis[(4-(2-hydroxyethoxy)phenyl)]fluorene was added as a crystalline species, and the mixture was held at 70°C for 2 hours and then cooled to 20°C. The precipitated crystals were filtered and dried to give 78.0 g of white crystals 9,9-bis[(4-(2-hydroxyethoxy)phenyl)]fluorene (yield: 90.2%, purity: 99.2%). The melting point of the resulting product was 163 °C.

122-99-6
784 suppliers
$8.00/100g

486-25-9
485 suppliers
$5.00/25g

117344-32-8
284 suppliers
$6.00/5g
Yield:117344-32-8 94.5%
Reaction Conditions:
with sulfuric acid;ethanethiol for 12 h;Reflux;Reagent/catalyst;
Steps:
1-6
Add 150 grams of 9-fluorenone, 400 grams of phenoxyethanol, 7.5 grams of ethyl mercaptan, 375 grams of ethyl cyclohexane, and 4.5 grams of concentrated sulfuric acid into a 2 liter reaction flask. Stir and heat to reflux. While reacting, reflux and separate water The reaction was stopped after 12 hours. Add 120 grams of water, cool and crystallize, stir at 05 for 2 hours, filter, rinse the filter cake with pure water until the pH of the rinse solution is neutral, and dry the filter cake to obtain 9,9-bis[4-( 2-hydroxyethoxy)phenyl]fluorene is 344.9 g, yield is 94.5%, content is 99.3%.
References:
CN112142574,2020,A Location in patent:Paragraph 0036-0060
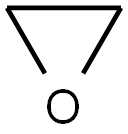
75-21-8
241 suppliers
$39.10/1mL

3236-71-3
421 suppliers
$6.00/5g

117344-32-8
284 suppliers
$6.00/5g
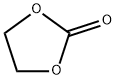
96-49-1
530 suppliers
$6.00/100g

3236-71-3
421 suppliers
$6.00/5g

117344-32-8
284 suppliers
$6.00/5g

122-99-6
784 suppliers
$8.00/100g
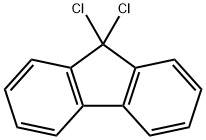
25023-01-2
65 suppliers
$57.00/1g

117344-32-8
284 suppliers
$6.00/5g
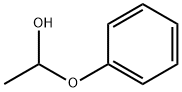
56101-99-6
1 suppliers
inquiry

486-25-9
485 suppliers
$5.00/25g
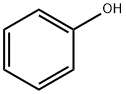
108-95-2
770 suppliers
$14.00/25g

117344-32-8
284 suppliers
$6.00/5g
