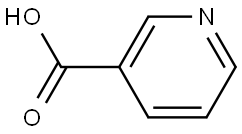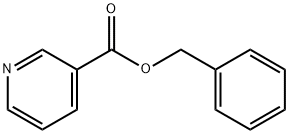
Benzyl nicotinate synthesis
- Product Name:Benzyl nicotinate
- CAS Number:94-44-0
- Molecular formula:C13H11NO2
- Molecular Weight:213.23
93-60-7
100-51-6

94-44-0
1. Catalyst Preparation: Lanthanum nitrate hexahydrate (La(NO3)3-6H2O, 17.3 mg, 0.04 mmol) and tri-n-octylphosphine (90% purity, 40 μL, 0.08 mmol) were added to a Soxhlet reflux vessel containing degreased cotton wool and 2.0 g of dried granular molecular sieve 5A. Dimethyl carbonate (8 mL), dehydrated by distillation, was added and stirred for 1 to 2 min at room temperature. The mixture was heated under heated reflux conditions (bath temperature 110°C) for 1 hour. Cool to room temperature, distill under reduced pressure to remove the solvent, and dry at room temperature for 1 hour at 5 torr or less to obtain the catalyst. 2. Reaction Procedure: In a reaction vessel, n-hexane (8 mL) was added sequentially as solvent, 4-nitrobenzoate (4.0 mmol) as carboxylic acid ester, and benzyl alcohol (4.0 mmol) as primary alcohol. The reaction was immediately heated to reflux conditions (bath temperature: 90 °C). The progress of the reaction was monitored by TLC and the completion of the reaction was confirmed after 5 hours. Cool to room temperature, add a small amount of water (0.3 to 0.5 mL) and stir for 5 min at room temperature to terminate the reaction. The reaction mixture was dried with magnesium sulfate, filtered and the filtrate was concentrated. The product was purified by silica gel column chromatography (hexane: ethyl acetate) in 99% yield. 3. For Examples 25-29 and Comparative Examples 10-14, the operation was the same as in Example 24, with only the carboxylate and reaction time changed. In Example 30 and Comparative Example 15, methyl benzoate was used as the carboxylic acid ester, cyclohexanol was used as the secondary alcohol, and the reaction time was adjusted. In Examples 26, 27, 29 and Comparative Examples 11, 12, 15, the amount of lanthanide compound and ligand was increased. The results are summarized in Table 4, including the results of Example 24 and Contrast Ratio 9.
Yield:94-44-0 99%
Reaction Conditions:
with lanthanum(III) isopropoxide;2-(2-methoxyethoxy)ethyl alcohol at 110; for 6 h;Molecular sieve;Reagent/catalyst;
Steps:
12
General procedure: Lanthanum nitrate hexahydrate (La (NO 3) 3 .6H 2 0, 17.3 mg, 0.04 mmol) and nitrous oxide were added to a Soxhlet reflux vessel containing absorbent cotton and 2.0 g of dried pelletized molecular sieves 5 A (MS 5 A) , Tri-n-octylphosphine (90% purity, 40 μL, 0.08 mmol) and dimethyl carbonate dehydrated by distillation (8 mL) were placed and stirred at room temperature for 1 to 2 minutes. The resulting mixture was heated under heating reflux conditions (bath temperature of 110 ° C.) for 1 hour. The mixed solution was cooled to room temperature, the solvent component was distilled off under reduced pressure, and the mixture was dried at room temperature under 5 Torr or less for 1 hour to prepare a catalyst. In the reaction vessel, n-hexane (8 mL) as a solvent, 4-nitrobenzoic acid ester (4.0 mmol) as a carboxylic acid ester and benzyl alcohol (4.0 mmol) as a primary alcohol were added in this order. Immediately, the reactor was heated to reflux condition (bath temperature: 90 ° C.). Refluxing was continued while appropriately checking the progress of the reaction by TLC, and after 5 hours, the completion of the reaction was confirmed by TLC. Thereafter, the reaction mixture was cooled to room temperature, a small amount of water (0.3 to 0.5 mL) was added, and the reaction was stopped by stirring at room temperature for 5 minutes. The reaction mixture was dried over magnesium sulfate, filtered, and the filtrate was concentrated. The product was isolated from the concentrate by silica gel column chromatography (n-hexane: ethyl acetate). The yield was 99%.In Examples 25 to 29 and Comparative Examples 10 to 14, a product was obtained in the same manner as in Example 24, except that the compound shown in Table 4 was used as the carboxylic acid ester and the reaction time was changed. In addition, in Example 30 and Comparative Example 15, production was carried out in the same manner as in Example 24, except that methyl benzoate was used as the carboxylic acid ester, cyclohexanol as the secondary alcohol was used as the alcohol compound, and the reaction time was changed I got things. However, in Examples 26, 27 and 29 and Comparative Examples 11, 12 and 15, as shown in Table 4, the amounts of lanthanum compounds and ligands used were increased. The results are shown in Table 4 including the results of Example 24 and Comparative Example 9.
References:
JP5804472,2015,B2 Location in patent:Paragraph 0054; 0055; 0056

25983-73-7
0 suppliers
inquiry

94-44-0
270 suppliers
$9.00/5g

1345837-77-5
1 suppliers
inquiry

94-44-0
270 suppliers
$9.00/5g