Benzothiazole, 2-(1H-indol-3-yl)- synthesis
- Product Name:Benzothiazole, 2-(1H-indol-3-yl)-
- CAS Number:31224-76-7
- Molecular formula:C15H10N2S
- Molecular Weight:250.32
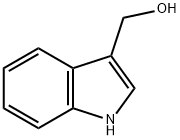
700-06-1
648 suppliers
$10.00/1g
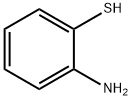
137-07-5
320 suppliers
$10.00/5g

31224-76-7
13 suppliers
$599.00/1g
Yield:31224-76-7 96%
Reaction Conditions:
with tetrabutylammonium bromide in N,N-dimethyl-formamide at 80; for 2.66667 h;
Steps:
2.6. General procedure for the synthesis of benzimidazoles or benzothiazoles in the presence of Pd(II)Cl2-BTP(at)MNPs catalyst
General procedure: A mixture of alcohol (1 mmol), 1,2-phenylenediamine or 2-aminothiophenol(1 mmol), Pd(II)Cl2-BTPMNPs (0.019 g, containing 0.09mol% Pd) and (1 mmol) tetrabutylammonium bromide (TBAB, 0.01 g)in DMF (5 mL) in a round-bottomed flask equipped with a condenser wasstirred at 80 °C. The progress of the reaction was monitored by TLC(eluent: n-Hexane/EtOAc, 4: 1 for benzimidazoles and n-Hexane/EtOAc, 6: 1 for benzothiazoles). The catalyst was separated by permanentmagnet and washed with EtOAc (10 mL). The crude product waspurified by recrystallization from EtOAc or EtOH to afford the purebenzimidazole. The benzothiazoles was obtained by recrystallizationfrom n-hexane/EtOAc (10: 1).
References:
Dehbanipour, Zahra;Zarnegareyan, Ali [Inorganic Chemistry Communications,2022,vol. 141,art. no. 109513]
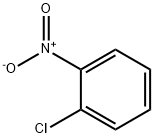
487-89-8
620 suppliers
$6.00/10g

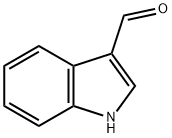
137-07-5
320 suppliers
$10.00/5g

31224-76-7
13 suppliers
$599.00/1g
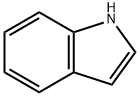
120-72-9
651 suppliers
$6.00/25g
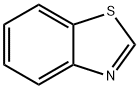
95-16-9
418 suppliers
$14.14/10gm:

31224-76-7
13 suppliers
$599.00/1g
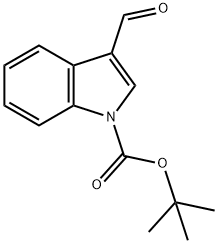
57476-50-3
119 suppliers
$6.00/1g

31224-76-7
13 suppliers
$599.00/1g