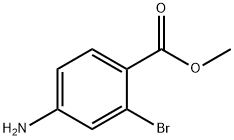
Methyl 4-amino-2-bromobenzoate synthesis
- Product Name:Methyl 4-amino-2-bromobenzoate
- CAS Number:98545-64-3
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06

100959-22-6

98545-64-3
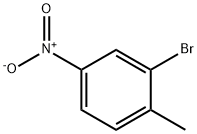
General procedure for the synthesis of methyl 4-amino-2-bromobenzoate from methyl 2-bromo-4-nitrobenzoate: To a solution of methyl 2-bromo-4-nitrobenzoate (4.5 g, 17.0 mmol) in ethyl acetate (100 mL) was added stannous chloride dihydrate (38.3 g, 0.17 mol). The reaction mixture was heated to reflux and stirred for 4 hours. Upon completion of the reaction, the mixture was poured into a mixture of saturated sodium bicarbonate solution (500 mL) and ethyl acetate (370 mL). The organic layer was separated, washed with saturated brine (300 mL) and dried with anhydrous magnesium sulfate. After concentration under reduced pressure, the residue was purified by silica gel column chromatography to afford methyl 4-amino-2-bromobenzoate as a solid (3.68 g, 94.2% yield).1H NMR (400 MHz, CDCl3): δ 7.75 (d, J = 8.5 Hz, 1H), 6.92 (d, J = 2.2 Hz, 1H), 6.57 (dd, J = 8.5,2.2 Hz, 1H), 4.04 (brs, 2H) and 3.86 (s, 3H) ppm; melting point: 96-98 °C.

100959-22-6
139 suppliers
$14.00/1g
32338-02-6
135 suppliers
$14.00/1g

98545-64-3
71 suppliers
$8.00/100mg
Yield: 96%
Steps:
6 Methyl 2-bromo-4-hydroxybenzoate (10)
Next, the nitro group of methyl 2-bromo-4-nitrobenzoate (6.38 g, 24.6 mmol) was chemoselectively reduced with 5 nCl2.2H2O (27.8 g, 123.2 mmol) in a manner similar to that described for compound 6 to deliver methyl 4-amino-2-bromobenzoate in 96.0% (5.41 g) yield as a yellow solid. 1H-NMR (CDCl3, 400 MHz) δ 7.74 (1H, d, J=8.8 Hz, Ar), 6.90 (1H, d, J=1.6 Hz, Ar), 6.55 (1H, dd, J=8.8 Hz, 1.6 Hz, Ar), 4.06 (2H, s, NH2), 3.83 (3H, s, OCH3); 13C-NMR (CDCl3, 100 MHz) δ 165.9, 150.4, 133.6, 124.1, 119.7, 119.6, 112.7, 51.8; MS (ESI) m/z Calcd for C8H8BrNO2 (M+): 229.0, Found: 230.1 (M+H+).
References:
UNIVERSITY OF MARYLAND, BALTIMORE;FLETCHER, Steven US2014/256817, 2014, A1 Location in patent:Page/Page column

100959-22-6
139 suppliers
$14.00/1g

98545-64-3
71 suppliers
$8.00/100mg

100959-22-6
139 suppliers
$14.00/1g
7439-89-6
463 suppliers
$10.00/25g

98545-64-3
71 suppliers
$8.00/100mg
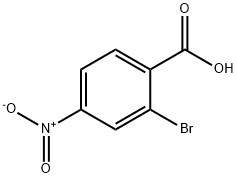
16426-64-5
160 suppliers
$5.00/250mg

98545-64-3
71 suppliers
$8.00/100mg
7745-93-9
293 suppliers
$5.00/5g

98545-64-3
71 suppliers
$8.00/100mg