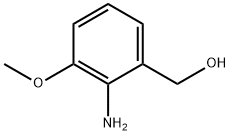
Benzenemethanol, 2-amino-3-methoxy- (9CI) synthesis
- Product Name:Benzenemethanol, 2-amino-3-methoxy- (9CI)
- CAS Number:205877-13-0
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
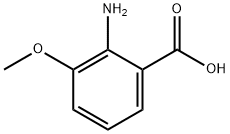
3177-80-8

205877-13-0
General method: 1.08 M THF solution of borane-tetrahydrofuran complex (24.3 mL, 26.2 mmol) of 2-amino-3-methoxybenzoic acid (1.5 g, 8.74 mmol) was slowly added dropwise to a solution of tetrahydrofuran (THF, 5 mL) at 0 °C under argon protection for a controlled time of 10 min. After the dropwise addition was completed, the reaction mixture was warmed up to 30 °C and stirred continuously for 1.5 hours. After completion of the reaction, the solution was cooled to 0 °C, a mixed solution of tetrahydrofuran and water (THF/H2O = 1:1, 60 mL) and potassium carbonate was added, followed by extraction with ether three times. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was recrystallized by ethyl acetate to give (2-amino-3-methoxy-phenyl)-methanol (1a, 1.2 g, 88% yield) as white needle-like crystals.

3177-80-8
261 suppliers
$9.00/1g

205877-13-0
60 suppliers
$307.00/1g
Yield:205877-13-0 100%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 1.66667 h;Inert atmosphere;
Steps:
87 5.1.4 2-Amino-6-chlorobenzyl alcohol (1a)
General procedure: To a solution of 6-chloroanthranilic acid (1.5 g, 8.74 mmol) in THF (5 mL) was added dropwise 1.08 M borane-tetrahydrofuran complex in THF (24.3 mL, 26.2 mmol) at 0 °C under an Ar atmosphere for 10 min. After 1.5 h with stirring at 30 °C, the solution was cooled at 0 °C, added aqueous THF (THF/H2O = 1:1, 60 mL) and potassium carbonate, and extracted with diethyl ether three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The residue was crystallized from AcOEt to give 1a (1.2 g, 88%) as a white needle crystal.
References:
Ida, Yoshihiro;Matsubara, Ayaka;Nemoto, Toru;Saito, Manabu;Hirayama, Shigeto;Fujii, Hideaki;Nagase, Hiroshi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 19,p. 5810 - 5831]
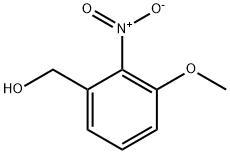
53055-04-2
40 suppliers
$120.00/0.25g

205877-13-0
60 suppliers
$307.00/1g
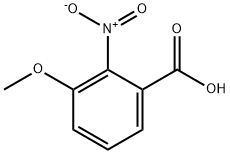
4920-80-3
205 suppliers
$14.00/1g

205877-13-0
60 suppliers
$307.00/1g