
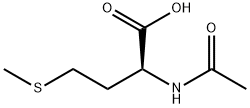
AC-MET-OME synthesis
- Product Name:AC-MET-OME
- CAS Number:35671-83-1
- Molecular formula:C8H15NO3S
- Molecular Weight:205.27
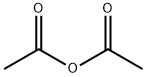
108-24-7
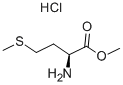
2491-18-1

35671-83-1
In a typical synthesis, 73 mL of acetic anhydride (780 mmol) was mixed with 63 mL of pyridine (780 mmol) in a round-bottomed flask and cooled in an ice bath.After 5-10 min, 8.6 g (100 mmol) of L-methionine methyl ester hydrochloride was added to the reaction mixture, which was then allowed to slowly rise to room temperature and stirred overnight. The following day, the reaction was quenched with cold water and extracted four times with 75 mL of dichloromethane. The combined organic phases were sequentially washed three times each with 1 M hydrochloric acid, saturated sodium bicarbonate solution and water. The organic layer was subsequently dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give a yellow oil, which crystallized on standing. The crude product was purified by recrystallization from ether at -20°C. The crystals were collected by vacuum filtration to give the product 19.3 g (94 mmol, 94% yield). The melting point was 41.7-42.4 °C. 1H NMR ([2H]-chloroform, TMS as internal standard, δ 0.0 ppm): δ 1.86-2.20 (m, 2H), δ 2.00 (s, 3H), δ 2.05 (s, 3H), δ 2.45-2.60 (m, 2H), δ 3.75 (s, 3H), δ 4.62-4.70 (m, 1H) , δ 6.13-6.19 (bd, 1H).

108-24-7
0 suppliers
$14.00/250ML

2491-18-1
282 suppliers
$6.00/5g

35671-83-1
73 suppliers
$50.00/100mg
Yield:35671-83-1 94%
Reaction Conditions:
with pyridine at 20;Cooling with ice;
Steps:
4.2.1. N-Ac-l-Met-OMe
In a typical reaction, 73 mL of acetic anhydride (780 mmol) and 63 mL pyridine (780 mmol) were combined in a round-bottomed flask and chilled on ice. After 5-10 min, 8.6 g (100 mmol) l-methionine methyl ester HCl were added and the reaction mixture was allowed to slowly return to room temperature overnight. The next morning, the reaction was quenched with cold water and extracted four times with 75 mL of methylene chloride. The extracts were then rinsed three times each with 1 M HCl, saturated sodium bicarbonate solution, and water. The extracts were then dried over MgSO4, filtered, and evaporated under reduced pressure, yielding a yellow oil which crystallized upon standing. The crude product was recrystallized in ethyl ether at -20 °C. Crystals (19.3 g, 94 mmol, 94%) were isolated by vacuum filtration. Mp = 41.7-42.4 °C. 1H NMR ([2H]-chloroform, TMS = 0.0 ppm): δ 1.86-2.20 (m, 2H), δ 2.00 (s, 3H), δ 2.05 (s, 3H), δ 2.45-2.60 (m, 2H), δ 3.75 (s, 3H), δ 4.62-4.70 (m, 1H), δ 6.13-6.19 (bd, 1H).
References:
Foster, Michael S.;Oldham, Charlie D.;May, Sheldon W. [Tetrahedron Asymmetry,2011,vol. 22,# 3,p. 283 - 293] Location in patent:experimental part

67-56-1
781 suppliers
$9.00/25ml
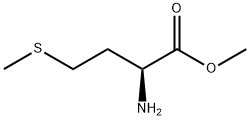
65-82-7
366 suppliers
$6.00/5g

35671-83-1
73 suppliers
$50.00/100mg
10332-17-9
21 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

35671-83-1
73 suppliers
$50.00/100mg

500872-34-4
2 suppliers
inquiry

35671-83-1
73 suppliers
$50.00/100mg

1115-47-5
424 suppliers
$6.00/25g

35671-83-1
73 suppliers
$50.00/100mg