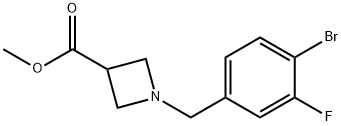
3-Azetidinecarboxylic acid, 1-[(4-bromo-3-fluorophenyl)methyl]-, methyl ester synthesis
- Product Name:3-Azetidinecarboxylic acid, 1-[(4-bromo-3-fluorophenyl)methyl]-, methyl ester
- CAS Number:950691-66-4
- Molecular formula:C12H13BrFNO2
- Molecular Weight:302.14

67-56-1
787 suppliers
$9.00/25ml
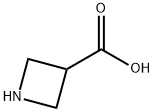
36476-78-5
435 suppliers
$6.00/1g
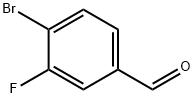
133059-43-5
236 suppliers
$6.00/1g
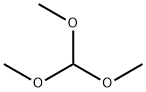
149-73-5
543 suppliers
$12.00/5g
![3-Azetidinecarboxylic acid, 1-[(4-bromo-3-fluorophenyl)methyl]-, methyl ester](/CAS/20210111/GIF/950691-66-4.gif)
950691-66-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3-azetidinecarboxylic acid;4-bromo-3-fluorobenzaldehyde;trimethyl orthoformatewith acetic acid in dichloromethane; for 0.25 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane; for 2 h;
Stage #3: methanolwith sulfuric acid for 18 h;Heating / reflux;
Steps:
3
Methyl 1-(4-bromo-3-fluorobenzyl)azetidine-3-carboxylate Azetidine-3-carboxylic acid (43 g, 421 mmol), 4-bromo-3-fluorobenzaldehyde (81.4 g, 401 mmol), methyl orthoformate (219 mL, 2005 mmol), and AcOH (34 mL, 601 mmol) were added to DCM (700 mL) at rt under an N2 atmosphere. The mixture was stirred for 15 min, at which point sodium triacetoxyborohydride (127 g, 601 mmol) was added portion-wise (exothermic). After 2 h, solvent swap with MeOH (257 g, 8019 mmol), and sulfuric acid (79 g, 802 mmol) was added slowly (exothermic). The mixture was heated at reflux for 18 h. Solvent was removed and the mixture was extracted using DCM and water. The organic layer was purified using a Biotage column (isopropanol/heptane), affording methyl 1-(4-bromo-3-fluorobenzyl)azetidine-3-carboxylate as a clear oil. MS (ESI) m/z: Calculated: 301.0; Observed: 302.0 (M++1).
References:
US2009/82331,2009,A1 Location in patent:Page/Page column 20