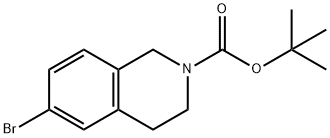
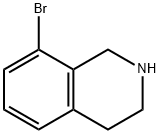
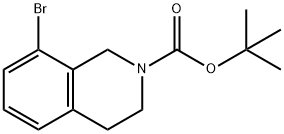
6-BROMO-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:6-BROMO-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:893566-74-0
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2
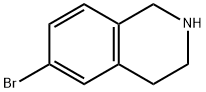
226942-29-6

24424-99-5

893566-74-0
Di-tert-butyl dicarbonate (3.52 g, 16.14 mmol) was slowly added to a solution of 6-bromo-1,2,3,4-tetrahydroisoquinoline (3.26 g, 15.37 mmol) in tetrahydrofuran (45 mL) at room temperature. The reaction mixture was stirred continuously for 15 h at room temperature. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The crude product was purified by silica gel column chromatography using solvent gradient elution (0→8% ethyl acetate/hexane) to afford the target compound tert-butyl 6-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate (5.05 g, 16.18 mmol, quantitative yield) as a colorless oil.1H NMR (300 MHz, CDCl3) δ: 1.49 (9H, s) ), 2.80 (2H, t, J = 5.9 Hz), 3.62 (2H, t, J = 5.9 Hz), 4.51 (2H, s), 6.97 (1H, d, J = 8.7 Hz), 7.28-7.32 (2H, m).
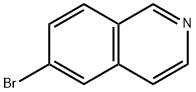
34784-05-9
327 suppliers
$5.00/1g

24424-99-5
867 suppliers
$13.50/25G

893566-74-0
109 suppliers
$28.00/250mg
Yield: 70.14%
Reaction Conditions:
Stage #1:6-bromo-isoquinoline with lithium triethylborohydride in tetrahydrofuran at 0 - 25; for 2 h;Inert atmosphere;
Stage #2:di-tert-butyl dicarbonate with sodium carbonate in tetrahydrofuran;water; pH=9 - 10 at 0 - 25; for 15 h;
Steps:
4.1 Step 1: Synthesis of Compound WX135-2
Under a nitrogen atmosphere, at 0° C., to a solution of WX135-1 (2.00 g, 9.61 mmol) in tetrahydrofuran (20.00 mL) was added lithium triethylborohydride (1 M, 42.28 mL) dropwise, and the system was reacted at 25° C. for 2 hours. After the reaction was complete, the system was firstly adjusted to pH=2-3 with 1M diluted hydrochloric acid, and then adjusted to pH=9-10 with sodium carbonate solution. At 0° C., to the system was added Boc2O (4.19 g, 19.22 mmol, 4.42 mL), and the reaction mixture was stirred at 25° C. for 15 hours. After the reaction was complete, at 25° C., to the reaction system was added 100 mL water. The system was diluted with 100 mL ethyl acetate, and extracted with ethyl acetate (100 mL×3). The organic layers were combined, washed with brine (150 mL×2), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography (petroleum ether:ethyl acetate=100:1-50:1) to afford Compound WX135-2 (2.42 g, 70.14% yield), colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.33-7.29 (m, 2H), 6.99 (br d, J=8.0 Hz, 1H), 4.52 (s, 2H), 3.63 (br s, 2H), 2.81 (br t, J=5.6 Hz, 2H), 1.50 (s, 9H).
References:
Medshine Discovery Inc.;He, Haiying;Jiang, Zhigan;Shi, Weihua;Xia, Jianhua;Li, Jian;Chen, Shuhui US2019/375744, 2019, A1 Location in patent:Paragraph 0132; 0133

226942-29-6
154 suppliers
$19.00/250mg

24424-99-5
867 suppliers
$13.50/25G

893566-74-0
109 suppliers
$28.00/250mg

215798-19-9
113 suppliers
$16.00/100mg

24424-99-5
867 suppliers
$13.50/25G

893566-74-0
109 suppliers
$28.00/250mg
164148-92-9
147 suppliers
$11.00/100mg

893566-74-0
109 suppliers
$28.00/250mg

226942-29-6
154 suppliers
$19.00/250mg

24424-99-5
867 suppliers
$13.50/25G
75416-51-2
93 suppliers
$24.00/100mg
893566-75-1
73 suppliers
$11.00/100mg

893566-74-0
109 suppliers
$28.00/250mg