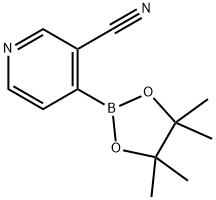
3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE synthesis
- Product Name:3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE
- CAS Number:878194-92-4
- Molecular formula:C12H15BN2O2
- Molecular Weight:230.07
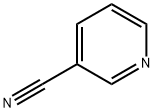
100-54-9
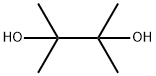
76-09-5
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
In a dry 500 mL three-necked flask protected by nitrogen, 2,2,6,6-tetramethylpiperidine (57.6 mmol) was dissolved in anhydrous tetrahydrofuran (200 mL), and after the reaction system was cooled to -10 °C, n-butyllithium (57.6 mmol, 2.5 M hexane solution) was added slowly and dropwise over a period of 2 minutes. After the dropwise addition, stirring was continued for 10 min, followed by cooling the reaction system to -78 °C. Triisopropyl borate (65.3 mmol) was added within 2 min at -78 °C and stirred for 5 min at this temperature. Then, 3-cyanopyridine (48 mmol) dissolved in anhydrous tetrahydrofuran (50 mL) was added with a dropwise addition time of 5 minutes. The reaction mixture was slowly warmed to room temperature in a dry ice bath and stirred overnight. Upon completion of the reaction, the reaction was quenched with glacial acetic acid (67.2 mmol) followed by the addition of pinacol (72 mmol). Stirring was continued for 2 hours at room temperature. The reaction mixture was transferred to a dispensing funnel, diluted by adding dichloromethane (75 mL) and washed with 10% w/v aqueous potassium dihydrogen phosphate solution (4 x 60 mL). The aqueous phase was back-extracted once with dichloromethane (15 mL), the organic phases were combined and dried over anhydrous magnesium sulfate. The solvent was concentrated under reduced pressure to afford the target product 3-cyano-4-pyridineboronic acid pinacol ester (3b) as a yellow solid in 18% yield with a melting point of 88°C. The product was analyzed by IR, 1H NMR and 1H NDT. The product was characterized by IR, 1H NMR and 13C NMR to confirm the structure.

100-54-9
568 suppliers
$5.00/10g

76-09-5
392 suppliers
$8.19/250mg
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
79 suppliers
$44.00/50 mg
Yield:878194-92-4 18%
Reaction Conditions:
Stage #1:pyridine-3-carbonitrile with Triisopropyl borate;2,2,6,6-tetramethylpiperidinyl-lithium in tetrahydrofuran;hexane at -78 - 20;Inert atmosphere;
Stage #2: with acetic acid in tetrahydrofuran;hexane at 20;Inert atmosphere;
Stage #3:2,3-dimethyl-2,3-butane diol in tetrahydrofuran;hexane at 20; for 2 h;Inert atmosphere;
Steps:
4.3.1. 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinonitrile (3b)
In a dry 500 mL flask under N2 2,2,6,6-tetramethylpiperidine (57.6 mmol) was dissolved in dry THF (200 mL) and the mixture was cooled to -10 °C before n-BuLi (57.6 mmol, 2.5 M in n-hexane) was added over 2 min. The mixture was stirred for 10 min before cooling to -78 °C. At -78 °C, B(O-i-Pr)3 (65.3 mmol) was added over 2 min and stirred for 5 min at -78 °C before 3-cyanopyridine (48 mmol) dissolved in dry THF (50 mL) was added dropwise over 5 min. The reaction was left in the dry ice bath, allowed to reach room temperature overnight and quenched with glacial acetic acid (67.2 mmol) followed by addition of pinacol (72 mmol). The mixture was stirred for 2 h at room temperature and then transferred to a separating funnel with CH2Cl2 (75 mL) and washed with aqueous KH2PO4 (10 w/v %) (4×60 mL). The combined aqueous layers was back-extracted once with CH2Cl2 (15 mL), the combined organic phase was dried over MgSO4, and the solvents were evaporated to give cyanopyridineboronic ester 3b as a yellow solid with 18% yield; mp 88 °C; IR (KBr) ν 3097, 2968, 2930, 2238 (CN), 1618, 1522, 1476, 1464, 1421, 1385, 1373, 1337, 1201, 1113, 1046, 963, 882, 849, 778, 765, 714, 655, 578 cm-1; 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H, H2), 8.74 (d, J=4.6 Hz, 1H, H6), 7.70 (d, J=4.6 Hz, 1H, H5), 1.34 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 153.3, 152.1, 129.3, 117.1, 114.2, 85.8, 83.1, 25.0.
References:
Rochais, Christophe;Yougnia, Rodrigue;Cailly, Thomas;Sopková-De Oliveira Santos, Jana;Rault, Sylvain;Dallemagne, Patrick [Tetrahedron,2011,vol. 67,# 32,p. 5806 - 5810] Location in patent:experimental part
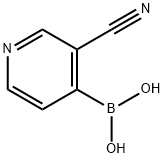
874290-89-8
62 suppliers
inquiry

76-09-5
392 suppliers
$8.19/250mg
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
79 suppliers
$44.00/50 mg

100-54-9
568 suppliers
$5.00/10g
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
79 suppliers
$44.00/50 mg