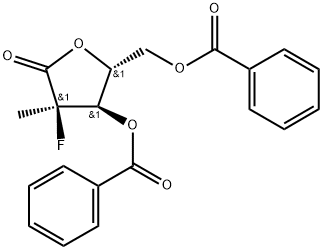
((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl)methyl benzoate synthesis
- Product Name:((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl)methyl benzoate
- CAS Number:874638-80-9
- Molecular formula:C20H17FO6
- Molecular Weight:372.35
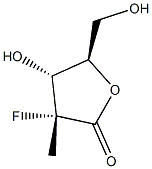
879551-04-9
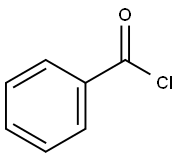
98-88-4

874638-80-9
(3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one (60 mg, 0.16 mmol) was dissolved in anhydrous pyridine (1 mL) and benzoyl chloride (0.3 mL) was added slowly. The reaction mixture was stirred at room temperature for 20 min, then water (1 mL) was added and stirring was continued for 20 min. The reaction solution was diluted with ethyl acetate (5 mL) and washed sequentially with water (2 mL) and 1M HCl (2 mL x 3). The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=10:1) to afford ((2R,3R,4R)-3-(benzyloxy)-4-fluoro-4-methyl-5-oxo-tetrahydrofuran-2-yl)methyl benzoate (118 mg, 87% yield) as a white solid.1H NMR (CDCl3) δ (ppm): 8.08 (m, 2H. Ar-H), 7.99 (m, 2H, Ar-H), 7.63 (m, 1H, Ar-H), 7.58 (m, 1H, Ar-H), 7.49 (m, 2H, Ar-H), 7.43 (m, 2H, Ar-H), 5.51 (dd, 1H, J=7.2,17.6Hz, H-3), 5.00 (m, 1H, H-4). 4.78 (dd, 1H, J=3.6,12.8Hz, H-5), 4.59 (dd, 1H, J=5.2,12.8Hz, H-5'), 1.75 (d, 3H, J=23.6Hz, CH3-2).

93635-76-8
142 suppliers
$10.00/5g

874638-80-9
334 suppliers
$5.00/1g
Yield:-
Steps:
Multi-step reaction with 4 steps
1: sulfur(VI) fluoride; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine trishydrofluoride / acetonitrile / 5 h / -15 - 90 °C
2: 2,2-dimethoxy-propane; hydrogenchloride; lithium hydroxide monohydrate / tetrahydrofuran / 3 h / 20 °C
3: hydrogenchloride; lithium hydroxide monohydrate / ethanol / 21 h / 20 °C
4: pyridine / 0.5 h / 20 °C / Cooling with ice
References:
US2013/72699,2013,A1
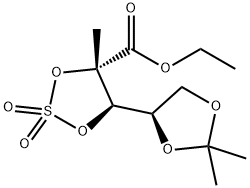
879551-01-6
11 suppliers
inquiry

874638-80-9
334 suppliers
$5.00/1g

98-88-4
604 suppliers
$10.00/5g

874638-80-9
334 suppliers
$5.00/1g
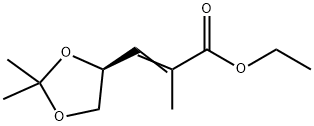
130606-67-6
5 suppliers
inquiry

874638-80-9
334 suppliers
$5.00/1g