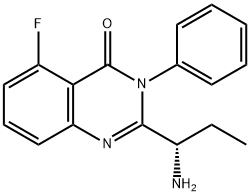
(S)-2-(1-aMinopropyl)-5-fluoro-3-phenylquinazolin-4(3H)-one synthesis
- Product Name:(S)-2-(1-aMinopropyl)-5-fluoro-3-phenylquinazolin-4(3H)-one
- CAS Number:870281-86-0
- Molecular formula:C17H16FN3O
- Molecular Weight:297.33
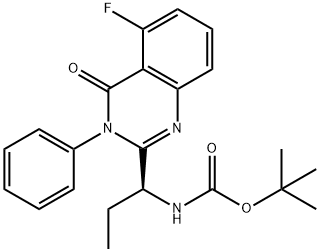
870281-85-9

870281-86-0
The general procedure for the synthesis of (S)-2-(1-aminopropyl)-5-fluoro-3-phenyl-4(3H)-quinazolinone from tert-butyl (S)-(1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)carbamate was carried out as follows: the (S)-(1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl )propyl)carbamic acid tert-butyl ester (220 mg, 0.55 mmol) was dissolved in ethyl acetate (2 mL), followed by a one-time addition of an ethyl acetate solution of hydrogen chloride (2.5 mL, 3.88 M) at room temperature. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the resulting suspension was dissolved in water (20 mL) and the aqueous phase was extracted with ethyl acetate (20 mL) and the pH of the aqueous phase was adjusted with sodium carbonate powder to 8. Subsequently, the aqueous phase was further extracted with ethyl acetate (20 mL x 3). All organic phases were combined, washed with saturated saline (20 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford (S)-2-(1-aminopropyl)-5-fluoro-3-phenyl-4(3H)-quinazolinone as a white powder (163 mg, 100% yield).

870281-85-9
97 suppliers
$29.00/1g

870281-86-0
123 suppliers
$23.00/100mg
Yield:870281-86-0 100%
Reaction Conditions:
with hydrogenchloride in water;ethyl acetate at 20;
Steps:
39.4 Step 4) (S) -2- (1-Aminopropyl) -5-fluoro-3-phenylquinazolin-4 (3H) -one
The compound (S) - (1- (5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl) propyl) carbamic acidTert-butyl ester (220 mg, 0.55 mmol) was dissolved in EtOAc (2 mL) and then a solution of hydrogen chloride in ethyl acetate (2.5 mL, 3.88 M) was added in one portion at room temperature. The resulting mixture was stirred at rt overnight, the resulting suspension was dissolved in water (20 mL) and the aqueous phase was extracted with EtOAc (20 mL) and Na2CO3 powder adjusted to pH = 8 and extracted with EtOAc (20 mL x 3 ). The combined organic phases were washed with brine (20 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the title compound as a white powder (163 mg, 100%).
References:
CN104513235,2017,B Location in patent:Paragraph 1158; 1159; 1160; 1161
![(S)-([1-(2-fluoro-6-nitro-benzoyl)-phenyl-aMinocarbonyl]-propyl)-carbaMic acid tert-butyl ester](/CAS/20150408/GIF/870281-84-8.gif)
870281-84-8
42 suppliers
inquiry

870281-86-0
123 suppliers
$23.00/100mg
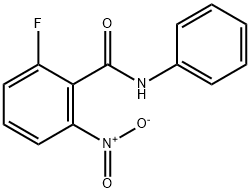
870281-83-7
75 suppliers
$8.00/250mg

870281-86-0
123 suppliers
$23.00/100mg
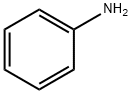
62-53-3
689 suppliers
$10.00/1g

870281-86-0
123 suppliers
$23.00/100mg
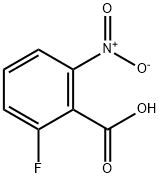
385-02-4
212 suppliers
$16.00/5g

870281-86-0
123 suppliers
$23.00/100mg