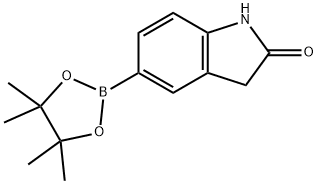
5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL) INDOLIN-2-ONE synthesis
- Product Name:5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL) INDOLIN-2-ONE
- CAS Number:837392-64-0
- Molecular formula:C14H18BNO3
- Molecular Weight:259.11
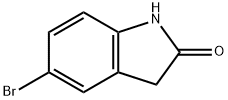
20870-78-4
331 suppliers
$7.00/1g
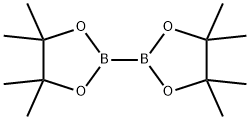
73183-34-3
591 suppliers
$6.00/5g

837392-64-0
125 suppliers
$130.46/1G
Yield: 93.8%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 85; for 15 h;
Steps:
58.A 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)indolin-2-one
EXAMPLE 58A 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)indolin-2-one Under N2, 5-Bromoindolin-2-one (Aldrich, 2.11 g, 10.0 mmol) was coupled with bis(pinacolato)dibon (Frontier Scientific, 3.05 g, 12 mmol) in the presence of KOAc (Aldrich, 1.50 g, 15.0 mmol) under the catalysis of PdCl2(dppf).CH2Cl2 (Aldrich, 163 mg, 0.2 mmol) in anhydrous dioxane (Aldrich, 50 mL) at 85° C. for 15 hours. After the reaction was completed, it was cooled down to ambient temperature and diluted with EtOAc (100 mL). The mixture was then washed with brine (2*10 mL) and concentrated. The residue was purified with chromatography on silica gel (EtOAc/hexanes, v. 1:1, Rf=0.5) to provide the title compound (2.43 g, yield, 93.8%). 1H NMR (300 MHz, CD3OD) δ 1.24 (s, 12H), 3.51 (s, 2H), 6.88 (d, J=8.5 Hz, 1H), 7.52-7.75 (m, 2H) ppm. MS (DCI/NH3): m/z 260 (M+H)+.
References:
Ji, Jianguo;Li, Tao;Lynch, Christopher L.;Gopalakrishnan, Murali US2008/45539, 2008, A1 Location in patent:Page/Page column 30-31
59-48-3
481 suppliers
$5.00/1g

837392-64-0
125 suppliers
$130.46/1G
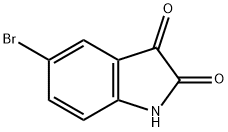
87-48-9
292 suppliers
$10.00/5g

837392-64-0
125 suppliers
$130.46/1G