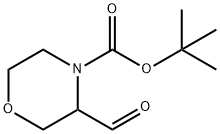
3-FORMYL-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-FORMYL-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:833474-06-9
- Molecular formula:C10H17NO4
- Molecular Weight:215.25

473923-56-7
98 suppliers
$19.00/100mg

833474-06-9
61 suppliers
$137.00/100mg
Yield: 84%
Reaction Conditions:
Stage #1:tert-Butyl 3-(hydroxymethyl)-4-morpholinecarboxylate with oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 2.25 h;
Stage #2: with triethylamine in dichloromethane at -78 - 20; for 1.5 h;
Steps:
91
INTERMEDIATE 91fert-Butyl 3 -formylmorpholine-4-carboxylateTo a stirred solution of oxalyl chloride (3.73 g, 2.56 mL, 29.00 mmol) in DCM (80 mL) at -780C was added DMSO (4.93 g, 4.47 mL, 63.00 mmol) and, after 15 minutes, a solution of Intermediate 90 (5.70 g, 26.26 mmol) in DCM (50 mL) was added. The reaction mixture was then stirred at -780C for a further 2 h. NEt3 (13.12 g, 18.71 mL, 129.70 mmol) was added and the reaction mixture was stirred at -780C for 30 minutes, warmed to r.t. and stirred for a further 1 h. The reaction mixture was then concentrated in vacuo and the residue partitioned between water (200 mL) and EtOAc (200 mL). The aqueous fraction was extracted with EtOAc (2 x 200 mL) and the combined organic fractions were washed with brine (300 mL), dried (MgSO4), filtered and the solvents removed in vacuo to give the title compound (4.80 g, 84%) as a pale yellow solid that was used crude. δH (CDCl3) 9.58 (IH, s), 4.31 (2H5 m), 3.62 (2H, br. m), 3.41 (IH, br. m), 3.11 (IH, br. s), 2.93 (IH, br. m), 1.40 (9H, s).
References:
UCB S.A. WO2006/114606, 2006, A1 Location in patent:Page/Page column 70
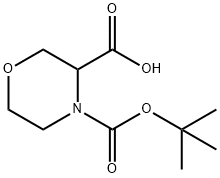
212650-43-6
160 suppliers
$46.00/250mg

833474-06-9
61 suppliers
$137.00/100mg

106910-79-6
89 suppliers
$50.00/250 mg

833474-06-9
61 suppliers
$137.00/100mg
110167-20-9
69 suppliers
$45.00/50mg

833474-06-9
61 suppliers
$137.00/100mg
24424-99-5
867 suppliers
$13.50/25G

833474-06-9
61 suppliers
$137.00/100mg