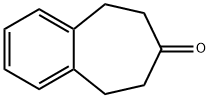
8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one synthesis
- Product Name:8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one
- CAS Number:37949-03-4
- Molecular formula:C11H12O
- Molecular Weight:160.21
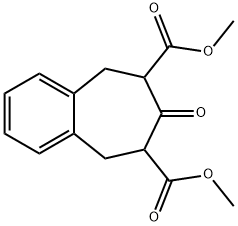
24790-66-7
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
The general procedure for the synthesis of 8,9-dihydro-5H-benzo[7]roten-7(6H)-ones from the compound (CAS:24790-66-7) was as follows: first, 3 5,6,8,9-tetrahydro-benzocyclohepten-7-one (compound of formula II) was prepared. A mixture of isomers of dimethyl 7-oxo-5,6,8,9-tetrahydrobenzocycloheptene-6,8-dicarboxylate (80 g, obtained according to Preparation 2) was dissolved in a mixture of aqueous 3M sulfuric acid (300 mL) and acetonitrile (50 mL) under argon protection and refluxed overnight. Upon completion of the reaction, the mixture was diluted with ether, neutralized with 2M aqueous sodium hydroxide solution (3 x 300 mL), subsequently dried with magnesium sulfate and evaporated to dryness. The residue was distilled at 97-98 °C, 0.4-0.5 Torr to give colorless crystals (26.1 g, 84% yield, α,α'-dibromo-o xylene). The melting point of the product was 43-44 °C. The 1H-NMR (CDCl3) data were as follows: 7.23 (m, 4H, aromatic hydrogen); 2.91 (m, 2H, hydrogen in the C(6) and C(8) positions); 2.62 (m, 2H, hydrogen in the C(5) and C(9) positions).

24790-66-7
2 suppliers
inquiry
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
93 suppliers
$27.00/100mg
Yield:37949-03-4 84%
Reaction Conditions:
Stage #1: dimethyl 7-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-6,8-dicarboxylatewith sulfuric acid in water;acetonitrile;Inert atmosphere;
Stage #2: with sodium hydroxide in diethyl ether;water;acetonitrile;
Steps:
3
Preparation 3 5,6,8,9-tetrahydro-benzocyclohepten-7-one (compound having the formula II) The isomeric mixture of dimethyl 7-oxo-5,6,8,9-tetrahydrobenzocycloheptene-6,8-dicarboxylate (80 g) obtained according to preparation 2 is refluxed in a 3M aqueous sulphuric acid (300 mL) and acetonitrile (50 mL) solution overnight under argon. The mixture is diluted with diethyl ether, neutralized with a 2M aqueous sodium hydroxide solution (3*300 mL), dried on magnesium sulphate and evaporated to dryness. The residue is distilled off at 97-98° C. under 0.4-0.5 Torr to obtain colourless crystals (26.1 g, 84% from α,α'-dibromo-o-xylene). Melting point: 43-44° C. 1H-NMR (CDCl3): 7.23 (m, 4 Har); 2.91 (m, 2H-C(6), 2H-C(8)); 2.62 (m, 2H-C(5), 2H-C(9)).
References:
US2010/69504,2010,A1 Location in patent:Page/Page column 5
56613-15-1
0 suppliers
inquiry
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
93 suppliers
$27.00/100mg
4443-91-8
6 suppliers
inquiry
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
93 suppliers
$27.00/100mg
5200-96-4
3 suppliers
inquiry
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
93 suppliers
$27.00/100mg
33188-51-1
0 suppliers
inquiry
![8,9-Dihydro-5H-benzo[7]annulen-7(6H)-one](/CAS/GIF/37949-03-4.gif)
37949-03-4
93 suppliers
$27.00/100mg