
7-methoxyquinoline synthesis
- Product Name:7-methoxyquinoline
- CAS Number:4964-76-5
- Molecular formula:C10H9NO
- Molecular Weight:159.18
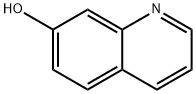
580-20-1

74-88-4

4964-76-5
General procedure for the synthesis of 7-methoxyquinoline from 7-hydroxyquinoline and iodomethane: 7-hydroxyquinoline (8 g, 55.11 mmol) was added to a solution of sodium hydride (5.5 g, 137.50 mmol, 60% pure) in N,N-dimethylformamide (150 mL). The reaction mixture was stirred in a water/ice bath at 0 °C for 1 hour. Subsequently, iodomethane (7.84 g, 55.23 mmol) was added and the reaction solution was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, the reaction was quenched by the addition of a water/ice mixture (700 mL) and extracted with ethyl acetate (3 x 200 mL). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography, using a petroleum ether solution of 6% ethyl acetate as eluent to give 7-methoxyquinoline as a red oil (5.5 g). The product was analyzed by LC-MS showing [M+H]+ peak of 160.0. 1H-NMR (300 MHz, CDCl3) data were as follows: δ 8.84-8.86 (m, 1H), 8.07-8.11 (m, 1H), 7.70-7.73 (t, J = 5.1 Hz, 1H), 7.44 (d, J = 2.4 Hz, 1H). 7.20-7.30 (m, 2H), 3.95 (s, 3H). Case No. BIOE0009-401-PC.

74-88-4
356 suppliers
$15.00/10g

4964-76-5
75 suppliers
$105.00/100mg
Yield: 99%
Reaction Conditions:
Stage #1:quinolin-7-ol with sodium hydride in N,N-dimethyl-formamide at 0; for 1 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
1.a
(a) 7-(Methyloxy)quinoline; A suspension of NaH (3.3g; 137.93mmol) in anhydrous DMF (160ml) was cooled to 0°C with stirring under argon. 7-Quinolinol (8g; 55.17mmol) dissolved in anhydrous DMF (320ml) was added and the mixture was stirred at 0°C under argon for Ih. The mixture was then allowed to warm to rt and MeI (7.8ml; 55.17mmol) was added and the reaction was stirred for Ih. Ice water was then added cautiously and the resulting mixture extracted with EtOAc (3 x 500ml). The organic layer from this extraction was then washed with water (400ml) and brine (400ml). The resulting organic layer was dried with MgSO4 and solvents removed to afford the desired compound (8.76g; 99%) MS (ES+) m/z 160 (MH+).
References:
GLAXO GROUP LIMITED WO2008/9700, 2008, A1 Location in patent:Page/Page column 32
19500-61-9
52 suppliers
inquiry

4964-76-5
75 suppliers
$105.00/100mg

580-20-1
202 suppliers
$12.00/1g

74-88-4
356 suppliers
$15.00/10g

4964-76-5
75 suppliers
$105.00/100mg
536-90-3
311 suppliers
$10.00/5g
56-81-5
1723 suppliers
$5.00/25g

4964-76-5
75 suppliers
$105.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4964-76-5
75 suppliers
$105.00/100mg