7-HYDROXYCOUMARIN-4-ACETIC ACID synthesis
- Product Name:7-HYDROXYCOUMARIN-4-ACETIC ACID
- CAS Number:6950-82-9
- Molecular formula:C11H8O5
- Molecular Weight:220.18
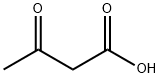
77-92-9
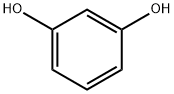
108-46-3

6950-82-9
GENERAL METHODS: Coumarin-4-acetic acid is a known compound (Manware et al., 2008). The reaction conditions for this compound were modified and optimized (Method A) to synthesize the analogues 2a-2f. These analogues were also synthesized by microwave-assisted methods (Method B). Method A: A mixture of citric acid (0.02 mol) and concentrated sulfuric acid (0.03 mol) was stirred at room temperature for 30 min. The mixture was placed in a boiling water bath to remove carbon monoxide (note: this needs to be done in a fume hood). Once the release of CO gas slowed down, the flask was removed from the water bath and left to stand for 15 minutes or until no more CO bubbles were produced in the reaction mixture. The reaction mixture was cooled to 10 °C and (un)substituted phenol (0.02 mol), pre-cooled to 10 °C, was added dropwise. The reaction mixture was stirred at room temperature for 48 h and then decanted onto ice. The precipitate was filtered and dissolved in saturated sodium bicarbonate solution. The solution was acidified to give intermediates 2a-2f. Method B: A mixture of citric acid (0.02 mol), concentrated sulfuric acid (0.03 mol), and (un)substituted phenol (0.02 mol) was placed in a microwave reactor and heated at 10% power for 4 min. The reaction mixture was poured onto crushed ice and processed according to the subsequent steps of Method A to obtain the target product. Yield of 7-hydroxycoumarin-4-acetic acid (2a): 67% for Method A and 78% for Method B; melting point: 170°C; IR (FT-IR) νmax (cm?1): 3210 (Ar C-H), 2924 (Al C-H), 1710 (C=O), 1701 (lactone C=O), 1458 (C=C), 1130 (C-O) , 1290 (C-OH); 1H-NMR (DMSO-d6, 400 MHz): δ 11.08 (1H, s, COOH), 7.41-6.98 (2H, m, H-5 and H-6), 6.69-6.23 (2H, m, H-8 and H-3), 4.93 (1H, dd, OH), 3.02-2.89 (2H, m , CH2); 13C-NMR (DMSO-d6, 100 MHz): δ 171.5 (C=O), 160.2 (C-2), 112.5 (C-3), 152.4 (C-4), 150.5 (C-8a), 135.2 (C-6), 124.8 (C-5), 122.9 (C-7), 120.1 (C-4a ), 104.1 (C-8), 37.0 (-CH2). HRMS (+ESI) [M+H]+: 221.0445 (calculated value), 221.0464 (measured value).

77-92-9
1702 suppliers
$5.00/10g

108-46-3
788 suppliers
$10.00/10g

6950-82-9
123 suppliers
$24.00/1g
Yield: 79%
Reaction Conditions:
Stage #1:citric acid with sulfuric acid at 250; for 1 h;
Stage #2:recorcinol with sulfuric acid at 0;
Steps:
a) Synthesis of compound (I)
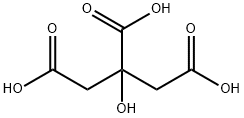
The hydrated citric acid (0.1 mol) was mixed with 28 ml of H2SO4(98%) under stirring for 60 min. at 250 °C, then the produced solutionis heated slowly to 75 °C with continuous quite stirring for 45 min. andthe solution turns to yellow color. This followed by quickly cooling ofthe yellow solution to 0 °C, to add both (0.08 mol) resorcinol and11.2 ml H2SO4 in three equivalent contents in the container of an icebath, and then it is kept overnight at 0 °C. After that, the previousproduct was poured into ice and the resulting crystalline white andtreated with NaHCO3 (200 ml 10%, w/v). The filtrate was acidified bythe addition of HCl (133 ml,10%v/v) to reaching the strong acidicmedium (pH 1.43). Finally, the produced compound was purified by itswashing with deionized water and dried in a thermal oven to give theprepared sample [34,35]. (Yield 79%and M.P 205 °C).
References:
Bedair, Mahmoud A.;Elsayed, Badr A.;Elsenety, Mohamed M.;Ibrahem, Ibrahem A. [Inorganic Chemistry Communications,2020,vol. 121]
542-05-2
491 suppliers
$6.00/10g

108-46-3
788 suppliers
$10.00/10g

6950-82-9
123 suppliers
$24.00/1g

108-46-3
788 suppliers
$10.00/10g

6950-82-9
123 suppliers
$24.00/1g

15991-13-6
25 suppliers
$45.00/50mg

6950-82-9
123 suppliers
$24.00/1g