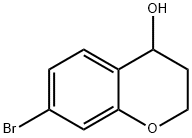
7-BROMOCHROMAN-4-OL synthesis
- Product Name:7-BROMOCHROMAN-4-OL
- CAS Number:18385-82-5
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

18442-22-3

18385-82-5
General procedure for the synthesis of 7-bromobenzodihydropyran-4-ol from 7-bromo-4-dihydrochromanone: sodium borohydride (545 mg, 14.4 mmol, 2.0 eq.) was added to a solution of 7-bromochroman-4-one (1.63 g, 7.2 mmol, 1.0 eq.) in methanol (10 mL), and the reaction mixture was stirred for 30 min at room temperature. Upon completion of the reaction, the solvent was removed by evaporation and the residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane = 1:2) to afford 7-bromobenzodihydropyran-4-ol (1.61 g, Yield: 98%) as a white solid.ESIMS (M+H-18): 211.0.1H NMR (400 MHz, CDCl3) δ: 7.17 (d, J = 8.0 Hz, 1H), 7.05-7.01 (m, 2H), 4.76-4.73 (m, 1H), 4.27-4.24 (m, 2H), 2.14-1.99 (m, 2H).

18442-22-3
139 suppliers
$12.00/250mg

18385-82-5
29 suppliers
$116.00/1g
Yield: 98%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;methanol at 20; for 0.5 h;
Steps:
96 Synthesis of 7-bromochroman-4-ol
[0331j To a solution of 7-bromochroman-4-one (1.63 g, 7.2 mmol, 1.0 equiv) in MeOH (10 mL) was added NaBH4 (545 mg, 14.4 mmol, 2.0 equiv) and then stirred at room temperature for 30 minutes. After evaporation of the solvent, the residue was purified by silica gel column (EtOAc/hexane=1 :2) to give 7-bromochroman-4-ol (1.61 g, yield: 98%) as a white solid. ESIMS (M+H-1 8) : 211.0. ‘H NMR (400 MHz, CDC13) 5: 7.17 (d, J = 8.0 Hz, 1H), 7.05-7.0 1 (m, 2H), 4.76-4.73 (m, 1H), 4.27-4.24 (m, 2H) 2.14-1.99 (m, 2H).
References:
BIOGEN IDEC MA INC.;SUNESIS PHARMACEUTICALS, INC.;HOPKINS, Brian, T.;MA, Bin;CHAN, Timothy, Raymond;SUN, Lihong;ZHANG, Lei;KUMARAVEL, Gnanasambandam;LYSSIKATOS, Joseph, P.;KOCH, Kevin;MIAO, Hua WO2015/89337, 2015, A1 Location in patent:Paragraph 0331
