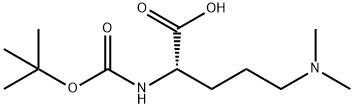
(S)-2-[(tert-Butoxycarbonyl)amino]-5-(dimethylamino)pentanoic acid synthesis
- Product Name:(S)-2-[(tert-Butoxycarbonyl)amino]-5-(dimethylamino)pentanoic acid
- CAS Number:65671-54-7
- Molecular formula:C12H24N2O4
- Molecular Weight:260.33
Yield:65671-54-7 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol;water at 25; under 2585.81 Torr; for 96 h;
Steps:
36
5-Benzyloxycarbonylamino-2(S)-tert-butoxycarbonylaminopentanoic acid (20 g, 54.6 mmoles) and 37% aqueous formaldehyde (130.1 mL, 131 mmoles) were dissolved in methanol-distilled water (1:1) (300 mL). 10% Pd-C (wet, 7 g) was added in portions under argon and the mixture was hydrogenated at 25° C. at 50 psi in a Parr hydrogenator for 4 days. The catalyst was filtered off through Celite and the latter was washed with methanol-distilled water (1:1). The combined filtrates were evaporated to dryness to give 2(S)-(+)-tert-butoxycarbonylamino-4-dimethylaminopentanoic acid (14.21 g, 100%): ESMS: m/z 261.0 (MH+); Found: C, 55.15; H, 8.97: N, 10.38; C12H24N2O4 requires: C, 55.36; H, 9.29; N, 10.96; δH (CDCl3) 1.40 (9H, s, COCC(CH3)3), 1.59 (1H, m, CHCH2CH2CH2N(CH3)2) 1.77 (3H, m, CHCH2CH2CH2N(CH3)2), 2.67 (6H, s, CHCH2CH2CH2N(CH3)2), 2.77 (1H, m, CHCH2CH2CH2N(CH3)2), 2,90 (1H, m, CHCH2CH2CH2N(CH3)2), 4.06 (1H, m, CHCH2CH2CH2N(CH3)2) and 5.68 ppm (1H, d, NH); δC (CDCl3) CH3: 28.5, 28.5, 28.5, 42.7, 42.7; CH2: 21.0, 30.2, 57.9; CH: 54.5; C, 78.9, 155.5, 176.5; [α]D25° C.+23.20 (c=0.51, MeOH).
References:
US2007/15774,2007,A1 Location in patent:Page/Page column 220