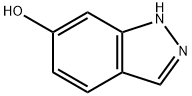
6-Hydroxyindazole synthesis
- Product Name:6-Hydroxyindazole
- CAS Number:23244-88-4
- Molecular formula:C7H6N2O
- Molecular Weight:134.14

6967-12-0

23244-88-4
General procedure for the synthesis of 6-hydroxyindazole from 6-aminoindazole: 6-aminoindazole (2.66 g, 20 mmol) was added to 20% dilute sulfuric acid and the reaction was carried out under microwave radiation (600 W, 170°C) for 1 hour. After completion of the reaction, the reaction solution was cooled and the pH was adjusted to 7 with 5% NaOH solution and stirred for 10 min to precipitate. The precipitate was recrystallized from water to give 1.5 g of 6-hydroxyindazole in 51% yield. Subsequently, 6-hydroxyindazole and 3-(1-(4-chlorobutyl)-4-piperidinyl)-6-fluorobenzisoxazole were used as raw materials and reacted according to step 4 in the method for the preparation of IV-1 to obtain 6-[4-(4-(4-(3-(6-fluorobenzisoxazolyl))-1-piperazinyl)-n-butoxy]-(1H)-indazole in 62% yield. Elemental analysis result: C23H23FN4O2 (calculated value %: C 66.99, H 5.88, N 14.20; measured value: C 66.71, H 5.80, N 13.89).1H NMR (DMSO-d6) data: δ 8.21-6.49 (6H, aryl ring-H), 4.18-4.19 (2H, piperazinyl-H), 3.87 ( m, 5H, O-CH2), 3.66-3.75 (2H, piperazine-H), 3.25-3.54 (m, 6H), 2.79 (t, J=8 Hz, 2H), 2.42 (t, J=8 Hz, 2H), 1.71-1.90 (m, 4H). Mass spectral data: m/z 394.

6967-12-0
201 suppliers
$9.00/1g

23244-88-4
169 suppliers
$28.00/1g
Yield:23244-88-4 87%
Reaction Conditions:
with sulfuric acid;water;sodium nitrite at 0 - 120;
Steps:
1 6.1.12. General procedure for the synthesis of 26 and 27
General procedure: To a suspension of corresponding amine (1 equiv) in H2O/98% H2SO4 (1:1) was added NaNO2 (1 equiv) slowly at 0 °C. After stirred at 120 °C for 2 h, the mixture was diluted with water (30 mL) and extracted with ethyl acetate (30 mL x 3). The combined organic layer was dried over Na2SO4 and concentrated. The residue was purified by silica gel column chromatography (dichloromethane/methanol) to afford the desired products 26 and 27. 6.1.12.1
H-Indazol-6-ol (26)
Orange solid (yield: 87%).
1H NMR (400 MHz, DMSO-d6): δ 12.55 (s, 1H), 9.56 (s, 1H), 7.85 (s, 1H), 7.51 (d, J = 8.8 Hz, 1H), 6.75 (s, 1H), 6.62 (dd, J = 8.8, 1.6 Hz, 1H).
References:
Dong, Yan;Li, Kehuang;Xu, Zhixiang;Ma, Haikuo;Zheng, Jiyue;Hu, Zhilin;He, Sudan;Wu, Yiyuan;Sun, Zhijian;Luo, Lusong;Li, Jiajun;Zhang, Hongjian;Zhang, Xiaohu [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 21,p. 6855 - 6868]
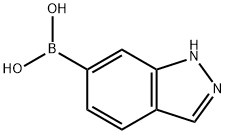
885068-10-0
153 suppliers
$13.00/250mg

23244-88-4
169 suppliers
$28.00/1g
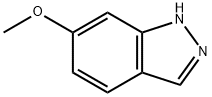
3522-07-4
131 suppliers
$26.00/100mg

23244-88-4
169 suppliers
$28.00/1g
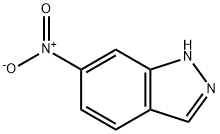
7597-18-4
307 suppliers
$17.00/25g

23244-88-4
169 suppliers
$28.00/1g
348-25-4
143 suppliers
$20.00/1g

23244-88-4
169 suppliers
$28.00/1g