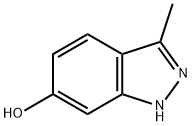
6-Hydroxy-3-methylindazole synthesis
- Product Name:6-Hydroxy-3-methylindazole
- CAS Number:201286-99-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

7746-29-4

201286-99-9
General procedure for the synthesis of 3-methyl-6-hydroxyindazole from 6-methoxy-3-methyl-1H-indazole: 6-methoxy-3-methyl-1H-indazole (620 mg, 3.82 mmol) was dissolved in dichloromethane (25 mL) and cooled to 0°C. Under stirring, a dichloromethane solution of boron tribromide (1 M, 17 mL) was slowly added. The ice bath was removed and the reaction mixture was allowed to gradually warm to room temperature and stirred overnight. Upon completion of the reaction, the reaction solution was quenched by slowly pouring it into a pre-cooled saturated aqueous sodium bicarbonate solution. The organic and aqueous phases were separated and the aqueous phase was extracted with ethyl acetate (3 x 25 mL). The organic phases were combined and concentrated to give the crude product. The crude product was purified by a Biotage fast chromatography system (40S silica gel column, eluent 45-60% acetone/heptane) to give 3-methyl-1H-indazol-6-ol (458 mg, 81% yield).

7746-29-4
76 suppliers
$26.00/250mg

201286-99-9
84 suppliers
$9.00/100mg
Yield:201286-99-9 81%
Reaction Conditions:
Stage #1: 6-methoxy-3-methyl-1H-indazolewith boron tribromide in dichloromethane at 20;Cooling with ice;
Stage #2: with water;sodium hydrogencarbonate in dichloromethane;Cooling with ice;
Steps:
14
To an ice cold solution of 6-methoxy-3-methyl-1 H-indazole (620 mg, 3.82 mmol) in CH2CI2 (25 mL) was added a solution of BBr3 in CH2CI2 (1 M, 17 ml_). The ice bath was removed and the reaction was allowed to warm to room temperature and stirred overnight. The solution was carefully quenched by slowly pouring into iced saturated aqueous NaHCO3. The phases were separated and the aqueous phase was extracted with EtOAc (3x). The combined organic extracts were concentrated and the crude material was purified Biotage (4OS column, 45-60% acetone/heptane) to provide 3-methyl-1 H-indazol-6-ol (458 mg, 81 %).
References:
WO2009/144554,2009,A1 Location in patent:Page/Page column 50

519158-44-2
2 suppliers
inquiry

201286-99-9
84 suppliers
$9.00/100mg

79173-62-9
99 suppliers
$60.00/100mg

201286-99-9
84 suppliers
$9.00/100mg
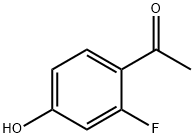
98619-07-9
154 suppliers
$8.00/1g

201286-99-9
84 suppliers
$9.00/100mg
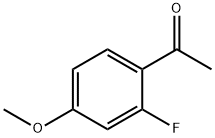
74457-86-6
261 suppliers
$6.00/5g

201286-99-9
84 suppliers
$9.00/100mg