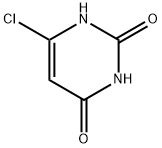
6-Chlorouracil synthesis
- Product Name:6-Chlorouracil
- CAS Number:4270-27-3
- Molecular formula:C4H3ClN2O2
- Molecular Weight:146.53

3764-01-0

4270-27-3
General procedure for the synthesis of 6-chloropyrimidine-2,4(1H,3H)-dione from 2,4,6-trichloropyrimidine: 2,4,6-trichloropyrimidine (10.21 g, 0.56 mol) was dissolved in an aqueous solution (100 mL) containing NaOH (8.9 g, 0.22 mol), which was stirred under refluxing conditions for 1 hour. Upon completion of the reaction, the pH of the reaction mixture was adjusted to 2-3 with concentrated hydrochloric acid. the mixture was placed in a refrigerator overnight to promote crystallization. Subsequently, the precipitate was collected by filtration, washed with cold water and dried under vacuum in the presence of phosphorus pentoxide. 6.43 g (0.54 mol, 97% yield) of 6-chlorouracil was finally obtained as a white crystalline solid. The spectral data of the product were in agreement with those reported in the literature [28] and [40]. Melting point > 280°C. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 12.00 (broad peak, 1H, NH), 11.09 (broad peak, 1H, NH), 5.66 (single peak, 1H, CH). IR (KBr, ν cm-1): 3095 (N-H stretching vibration), 1729,1709,1652 (C=O stretching vibration), 1616 (C=C stretching vibration), 1380 (C-N stretching vibration), 841 (N-H out-of-plane bending vibration), 790 (C-Cl stretching vibration).

3764-01-0
406 suppliers
$6.00/5g

4270-27-3
327 suppliers
$7.00/5g
Yield:4270-27-3 97%
Reaction Conditions:
Stage #1:2,4,6-trichloropyrimidine with sodium hydroxide in water for 1 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=2 - 3
Steps:
4.3.2. 6-Chlorouracil (6{1,1})
A solution of 2,4,6-trichloropyrimidine (11) (10.21 g, 0.56 mol) in water (100 mL) containing NaOH (8.9 g, 0.22 mol) was stirred under reflux for 1 h. The reaction mixture was adjusted to pH 2-3 with conc. aqueous HCl and was kept in a refrigerator overnight. The precipitate was collected by filtration, washed with cold water and dried in vacuo over phosphorus pentoxide. 6.43 g (0.54 mol, 97%) of 6-clorouracil (6{1,1}) were obtained as a white crystalline solid. Spectroscopic data was identical with those reported in the literature [28] and [40]. m.p. >280 °C. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 12.00 (br s, 1H, NH), 11.09 (br s, 1H, NH), 5.66 (s, 1H, CH). IR (KBr, ν cm-1): 3095 (N-H), 1729, 1709, 1652 (CO), 1616 (CC), 1380 (C-N), 841 (N-H), 790 (C-Cl).
References:
Puig-De-La-Bellacasa, Raimon;Gimenez, Laura;Pettersson, Sofia;Pascual, Rosalia;Gonzalo, Encarna;Este, Jose A.;Clotet, Bonaventura;Borrell, Jose I.;Teixido, Jordi [European Journal of Medicinal Chemistry,2012,vol. 54,p. 159 - 174] Location in patent:experimental part
6320-15-6
190 suppliers
$18.00/250mg

4270-27-3
327 suppliers
$7.00/5g
67-52-7
485 suppliers
$27.30/25g

4270-27-3
327 suppliers
$7.00/5g