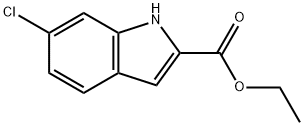
6-Chloroindole-2-carboxylic acid ethyl ester synthesis
- Product Name:6-Chloroindole-2-carboxylic acid ethyl ester
- CAS Number:27034-51-1
- Molecular formula:C11H10ClNO2
- Molecular Weight:223.66
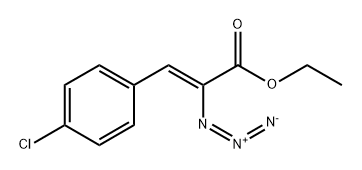
24513-05-1

27034-51-1
General method: 6a-d (9.2 mmol) was dissolved in toluene (150 mL) under nitrogen protection, rhodium(II) acetate dimer (203 mg, 0.46 mmol) was added, and the reaction was heated to reflux for 1-2 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure, and the resulting residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate, 25:1 to 18:1, v/v) to afford the target product ethyl 6-chloroindole-2-carboxylate (7a-d) in 51.7%-58.6% overall yields.
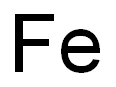
7439-89-6
460 suppliers
$10.00/25g

540523-98-6
3 suppliers
inquiry

27034-51-1
144 suppliers
$6.00/250mg
Yield: 50%
Reaction Conditions:
with hydrogenchloride in glacial HOAc;ethanol;dichloromethane
Steps:
105.2 4-{2-[1-Benzhydryl-6-chloro-2-(2-phenylmethanesulfonylamino-ethyl)-1H-indol-3-yl]-ethoxy}-benzoic acid
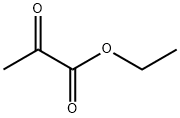
Step 2: To crude 3-(4-chloro-2-nitro-phenyl)-2-oxo-propionic acid ethyl ester (151 mmol) in ethanol:glacial HOAc (1:1, v/v, 560 mL) at rt was added iron powder (74.4 g) and the reaction mixture was stirred at reflux for 4 h. The mixture was filtered and evaporated to give a residue which was redistributed in dichloromethane/1N HCl. The organic layer was washed with 1N HCl, NaHCO3, and brine and dried. Evaporation followed by crystallization (DCM) gave 6-Chloro-1H-indole-2-carboxylic acid ethyl ester as a pale yellow solid (16.8 g, 50% over 2 steps).
References:
Wyeth US2003/144282, 2003, A1
617-35-6
558 suppliers
$5.00/10g
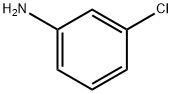
108-42-9
470 suppliers
$10.00/5g

27034-51-1
144 suppliers
$6.00/250mg

53590-46-8
86 suppliers
$44.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

27034-51-1
144 suppliers
$6.00/250mg

540523-98-6
3 suppliers
inquiry

27034-51-1
144 suppliers
$6.00/250mg
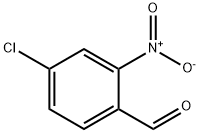
5551-11-1
164 suppliers
$17.00/1g
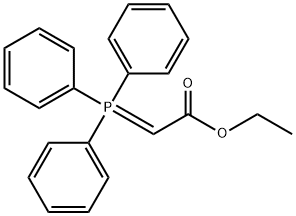
1099-45-2
373 suppliers
$6.00/5g

27034-51-1
144 suppliers
$6.00/250mg