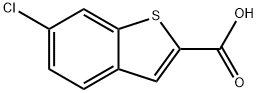
6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLIC ACID synthesis
- Product Name:6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLIC ACID
- CAS Number:26018-73-5
- Molecular formula:C9H5ClO2S
- Molecular Weight:212.65
![Methyl 6-chlorobenzo[b]thiophene-2-carboxylate](/StructureFile/ChemBookStructure22/GIF/CB9956809.gif)
104795-85-9

26018-73-5
1. To a solution of DMF (7 mL) containing 600 mg (3.23 mmol) of 4-chloro-2-nitrobenzaldehyde was added 559 mg (4.04 mmol) of potassium carbonate and 294 μL (3.23 mmol) of methyl thioglycolate sequentially at 0°C. The reaction mixture was stirred for 30 min. at 0°C followed by continued stirring at room temperature for 24 hours. The reaction mixture was stirred at 0°C for 30 minutes, followed by continued stirring at room temperature for 24 hours. Upon completion of the reaction, the mixture was poured into ice water, the precipitate was collected by filtration, and the precipitate was dissolved in ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate and concentrated to give 630 mg (86% yield) of methyl 6-chlorobenzo[b]thiophene-2-carboxylate as a white solid.1H NMR (CDCl3, 300 MHz): δ 3.95 (s, 3H), 7.88 (dd, J = 8.6, 1.9 Hz, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.85 (d, J = 8.6 Hz, 1H), 7.79 (d, J = 8.6 Hz, 1H). 7.85 (d, J = 1.9 Hz, 1H), 8.02 (s, 1H). 2. 6.95 mL of aqueous 1N LiOH was added to a 630 mg (2.78 mmol) solution of methyl 6-chlorobenzo[b]thiophene-2-carboxylate in THF (5 mL), and the reaction mixture was stirred for 4 h at room temperature. Upon completion of the reaction, the pH was adjusted to 2-3 with 1N HCl and subsequently extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated to give 579 mg (98% yield) of 6-chlorobenzo[b]thiophene-2-carboxylic acid as a white solid.MS (ISP): 211.0 (M-H)-. 3. 139 mg (0.65 mmol) of 6-chlorobenzo[b]thiophene-2-carboxylic acid was converted to 52 mg (0.14 mmol) of (6-chlorobenzo[b]thiophen-2-ylmethyl)-[2-(3,4-dichlorophenyl)ethyl]amine by reference to the method of Example S3 (Steps a to b), the product was a colorless liquid.MS (ISP): 370.0 (M+H )+.
![Methyl 6-chlorobenzo[b]thiophene-2-carboxylate](/StructureFile/ChemBookStructure22/GIF/CB9956809.gif)
104795-85-9
56 suppliers
$77.00/250mg

26018-73-5
80 suppliers
$19.00/100mg
Yield:26018-73-5 98%
Reaction Conditions:
Stage #1: methyl 6-chlorobenzo[b]thiophene-2-carboxylatewith lithium hydroxide;lithium hydroxide monohydrate in tetrahydrofuran at 20; for 4 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;lithium hydroxide monohydrate; pH=2 - 3;
Steps:
S13 Preparation of (6-chloro-benzo[b]thiophen-2-ylmethyl)-[2-(3,4-dichloro-phenyl)-ethyl]-amine
To a solution of 600 mg (3.23 mmol) of 4-chloro-2-nitro-benzaldehyde in DMF (7 ml) were added 559 mg (4.04 mmol) of potassium carbonate and 294 μl (3.23 mmol) of methyl thioglycolate at 0° C. The reaction mixture was stirred for 30 min at 0° C. and then for 24 h at RT. Then the mixture was poured into ice-water and the precipitate was collected by filtration and dissolved in ethyl acetate. The solution was dried (MgSO4) and concentrated to yield 630 mg (86%) of 6-chloro-benzo[b]thiophene-2-carboxylic acid methyl ester as a white solid. 1HNMR (CDCl3, 300 MHz): δ 3.95 (s, 3H), 7.88 (dd, J=8.6 and 1.9 Hz, 1H), 7.79 (d, J=8.6 Hz, 1H), 7.85 (d, J=1.9 Hz, 1H), 8.02 (s, 1H).To a solution of 630 mg (2.78 mmol) of 6-chloro-benzo[b]thiophene-2-carboxylic acid methyl ester in THF (5 ml) were added 6.95 ml of 1N LiOH-solution and the reaction mixture was stirred at RT for 4 h. The pH was then adjusted to 2-3 by addition of 1N HCl and the mixture was extracted with ethyl acetate. The combined organic extracts were washed with brine, dried (MgSO4) and concentrated to yield 579 mg (98%) of 6-chloro-benzo[b]thiophene-2-carboxylic acid as white solid. MS (ISP) 211.0 (M-H)-.In analogy to example S3 (steps a to b) 139 mg (0.65 mmol) of 6-chloro-benzo[b]thiophen-2-carboxylic acid were converted into 52 mg (0.14 mmol) of (6-chloro-benzo[b]thiophen-2-ylmethyl)-[2-(3,4-dichloro-phenyl)-ethyl]-amine. The product was obtained as colorless liquid. MS (ISP) 370.0 (M+H)+.
References:
US2007/185113,2007,A1 Location in patent:Page/Page column 13
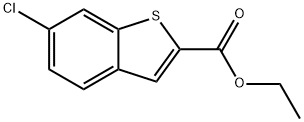
105191-53-5
25 suppliers
$489.00/5g

26018-73-5
80 suppliers
$19.00/100mg
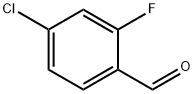
61072-56-8
288 suppliers
$14.00/5g

26018-73-5
80 suppliers
$19.00/100mg