
6-BROMO-BENZO[B]THIOPHENE synthesis
- Product Name:6-BROMO-BENZO[B]THIOPHENE
- CAS Number:17347-32-9
- Molecular formula:C8H5BrS
- Molecular Weight:213.09
![6-BROMO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/19075-58-2.gif)
19075-58-2
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
Part C. Preparation of 6-bromobenzo[b]thiophene. 6-Bromobenzo[b]thiophene-2-carboxylic acid (0.70 g, 2.73 mmol) from Part B was mixed with DBU (1.35 mL, 8.94 mmol) in DMA (6 mL) in a sealed tube and heated in a microwave reactor for 70 min at 200 °C. After completion of the reaction, the resulting dark solution was diluted with 1 M HCl (20 mL) and extracted with dichloromethane (2 x 20 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using dichloromethane as eluent to afford 6-bromobenzo[b]thiophene as an oil (0.484 g, 83% yield).
![Benzo[b]thiophene-3-ol, 6-bromo-2,3-dihydro-](/CAS/20200611/GIF/737802-11-8.gif)
737802-11-8
1 suppliers
inquiry
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
205 suppliers
$7.00/250mg
Yield:17347-32-9 95%
Reaction Conditions:
with boron trifluoride diethyl etherate in acetic acid at 20 - 120; for 0.0833333 h;
Steps:
139.D Example 139; N-[3-(1-Benzo[b]thiophen-6-yl-1-ethyl-propyl)-1H-indol-7-yl]-methanesulfonamide; D. Preparation of: 6-Bromo-benzo[b]thiophene
To a room temperature solution OF 6-BROMO-2, 3-dihydro-benzo [b] thiophen-3-ol (785 mg, 3.39 mmol) in HOAC (7 mL) is added boron trifluoride diethyl etherate (1. 44 G, 10.2 mmol) and the reaction mixture is placed into a 120 C oil bath. After 5 min, the reaction is cooled to room temperature and basified to-pH 11 using 2 M NaOH. The aqueous suspension is extracted with ET2O (2 X 200 mL) and the combined organic layer is dried (MGS04), filtered and concentrated to afford the sub-title compound (689 mg, 95%) as an off-white solid. RF 0. 70 (4: 1 HEX/ETOAC). mp 48-50 C. 'H NMR (300 MHz, CDCl3) 8 7.29 (d, J= 5.4 Hz, 1H), 7.41 (d, J= 5. 4 Hz, 1H), 7.46 (dd, J= = 1. 8,8. 5 Hz, 1H), 7.66 (d, J= 8.5 Hz, 1H), 8.01 (M, 1H).
References:
WO2004/67529,2004,A1 Location in patent:Page 162
![6-BROMO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/19075-58-2.gif)
19075-58-2
132 suppliers
$39.00/500mg
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
205 suppliers
$7.00/250mg

17347-29-4
3 suppliers
inquiry
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
205 suppliers
$7.00/250mg

6320-01-0
181 suppliers
$8.00/1g
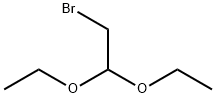
2032-35-1
485 suppliers
$5.00/10g
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
205 suppliers
$7.00/250mg
![4-Bromobenzo[b]thiophene](/CAS/GIF/5118-13-8.gif)
5118-13-8
272 suppliers
$8.00/1g

19296-69-6
8 suppliers
inquiry
![6-BROMO-BENZO[B]THIOPHENE](/CAS/GIF/17347-32-9.gif)
17347-32-9
205 suppliers
$7.00/250mg
![4-Bromobenzo[b]thiophene](/CAS/GIF/5118-13-8.gif)
5118-13-8
272 suppliers
$8.00/1g
