
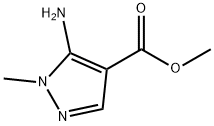
1-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:1-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER
- CAS Number:5952-93-2
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14
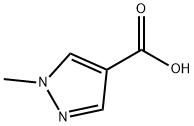
5952-92-1
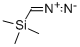
18107-18-1

5952-93-2
Step A: Trimethylsilylated diazomethane (12.0 mL, 24 mmol, 2.0 M hexane solution) was slowly added dropwise to a solution of 1-methyl-4-pyrazolecarboxylic acid (0.59 g, 4.7 mmol) in methanol (20 mL) at 0 °C through an addition funnel. The reaction mixture was stirred at the same temperature for 20 min followed by concentration. The crude product was partitioned between ethyl acetate (100 mL) and water (50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by fast column chromatography using hexane/ethyl acetate (4:1, v/v) as eluent to afford methyl 1-methyl-1H-pyrazole-4-carboxylate (0.50 g, 3.6 mmol, 76% yield) as a white solid. Mass spectrum (APCI-positive ion mode) m/z: [M+1]+ = 141.1.

51105-90-9
114 suppliers
$9.00/100mg

74-88-4
356 suppliers
$15.00/10g

5952-93-2
63 suppliers
$60.00/100mg
Yield:5952-93-2 90%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
131.1 first step 1-methyl-1H-pyrazole-4-carboxylate methyl ester
Methyl pyrazole-4-carboxylate 131a (5 g, 39.65 mmol) was dissolved in N,N-dimethylformamide (50 mL),Potassium carbonate (10.96 g, 79.29 mmol) and methyl iodide (6.19 g, 43.61 mmol) were sequentially added, and the reaction was stirred at room temperature for 1 hour.Water (50 mL) was added, extracted with ethyl acetate (50 mL×3), the organic phases were combined, washed successively with water (50 mL) and saturated sodium chloride solution (50 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure.The obtained residue was further separated and purified by silica gel column chromatography (eluent: system A),1-Methyl-1H-pyrazole-4-carboxylate methyl ester 131b (5.04 g) was obtained, yield: 90%.
References:
WO2022/57849,2022,A1 Location in patent:Page/Page column 166-167

5952-92-1
239 suppliers
$5.00/250mg
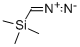
18107-18-1
210 suppliers
$33.00/5g

5952-93-2
63 suppliers
$60.00/100mg
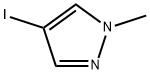
39806-90-1
232 suppliers
$9.00/5g

67-56-1
782 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

5952-93-2
63 suppliers
$60.00/100mg
110860-60-1
36 suppliers
$44.00/250mg

88398-85-0
20 suppliers
inquiry

5952-93-2
63 suppliers
$60.00/100mg

67-56-1
782 suppliers
$9.00/25ml

5952-92-1
239 suppliers
$5.00/250mg

5952-93-2
63 suppliers
$60.00/100mg