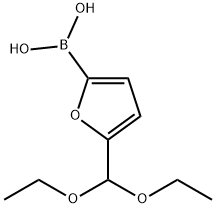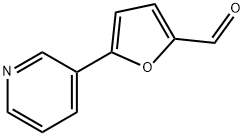
5-PYRIDIN-3-YL-2-FURALDEHYDE synthesis
- Product Name:5-PYRIDIN-3-YL-2-FURALDEHYDE
- CAS Number:38588-49-7
- Molecular formula:C10H7NO2
- Molecular Weight:173.17
Yield:38588-49-7 96%
Reaction Conditions:
with tetrabutylammomium bromide;potassium carbonate in methanol;water at 80; for 0.333333 h;Reflux;Suzuki Coupling;
Steps:
Synthesis of heterobiaryls 6a-k by Suzuki reactions(General method).
General procedure: A mixture of hetarylboronic acid 4a-d(1.2 mmol), aryl(hetaryl) bromide 5a-h or hetaryl chloride7a,b (1 mmol), Bu4NBr (3 mg, 1 mol %, for water-insolublearyl(hetaryl) halides 5b-g and 7a,b), and K2CO3 (346 mg,2.5 mmol) in 2 (5 ml) was heated to 80° and treated byadding 0.1-1 mol % of Pd-Ni(Co)-B-L (an aliquot of0.1 M solution of bimetallic catalyst in MeOH-H2Omixture). The reactor was fitted with a reflux condenserand placed in a hot silicone oil bath (150°). The reactionmixture was vigorously stirred at reflux until completeconversion of the starting materials was achieved. Thereaction progress was controlled by TLC method (eluenthexane-Et2O, 3:1). The amount of catalyst, reactionduration and yields of the target compounds 6a-k are listedin Table 4. In the case of the activated aryl bromides5a,b,d,f, the reaction was highly exothermic, therefore aneffective reflux condenser was essential for scaling up thissynthesis.After the reaction was complete, the mixture was dilutedwith H2O (10 ml), heated to 80°C, and filtered while hotthrough a Whatman autovial syringeless filter (pore size0.45 μm). The filtrate was diluted with 10-15 vol % ofEtOH, heated to ~50°C, stirred, and slowly acidified with5% HCl to pH 2-3. The resulting precipitate was easy tofilter, and analytically pure products 6a,h,k were obtainedwithout chromatographic purification. In the case of thewater-insoluble heterobiaryls 6b-g,i,j, the reaction mixturewas diluted with saturated solution of NaCl (10 ml) andextracted with Et2O or EtOAc (3×5 ml). The obtainedextract was dried over anhydrous Na2SO4, filtered througha silica gel layer, and the solvent was evaporated at reducedpressure. The residues in all cases were >99% pureproducts (according to the results of elemental analysis).Analytically pure samples were obtained by recrystallizationof heterobiaryls 6a-k from a minimal amount ofaqueous EtOH (10-20% 2) or by converting amines intothe respective hydrochlorides. The residual metal content inthe isolated heterobiaryls 6a-k did not exceed 1 ppmaccording to the results of atomic absorption spectrometry.
References:
Bumagin, Nikolay A.;Petkevich, Sergey K.;Kletskov, Alexey V.;Alekseyev, Roman S.;Potkin, Vladimir I. [Chemistry of Heterocyclic Compounds,2019][Khim. Geterotsikl. Soedin.,2019,vol. 556,# 6,p. 508 - 516,9]
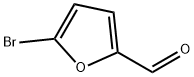
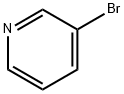
1899-24-7
268 suppliers
$13.43/500mgs:
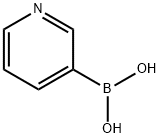
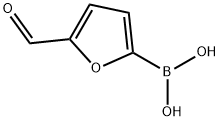
1692-25-7
484 suppliers
$5.00/1g

38588-49-7
18 suppliers
inquiry
![Pyridine, 3-[5-(1,3-dioxolan-2-yl)-2-furanyl]-](/CAS/20210305/GIF/342600-54-8.gif)
342600-54-8
0 suppliers
inquiry

38588-49-7
18 suppliers
inquiry