
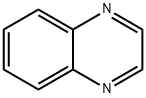
5-NITROQUINOXALINE synthesis
- Product Name:5-NITROQUINOXALINE
- CAS Number:18514-76-6
- Molecular formula:C8H5N3O2
- Molecular Weight:175.14

131543-46-9
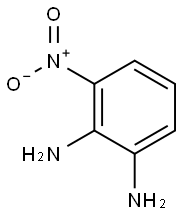
3694-52-8

18514-76-6
The general procedure for the synthesis of 5-nitroquinoxaline from the compound (CAS:131543-46-9) and 3-nitro-o-phenylenediamine was as follows: general method 108: Acetaldehyde (40% aqueous solution, 1.43 mL, 31.3 mmol) was added to a solution of 3-nitro-o-phenylenediamine (600 mg, 3.9 mmol) in ethanol (15 mL). The reaction mixture was heated to reflux at 85 °C for 2 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure, the residue was diluted with an appropriate amount of water and the aqueous phase was extracted with dichloromethane (DCM). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by column chromatography using dichloromethane/methanol (99:1, v/v) as eluent to afford the target compound 5-nitroquinoxaline (666 mg, 97% yield). Molecular weight (MW): 175.15. High performance liquid chromatography-mass spectrometry (HPLC-MS, Method C) analysis: [m/z]: 176.

131543-46-9
1 suppliers
inquiry

3694-52-8
165 suppliers
$15.00/250mg

18514-76-6
47 suppliers
inquiry
Yield:18514-76-6 97%
Reaction Conditions:
in ethanol at 85; for 2 h;
Steps:
108 General Procedure 108: 5-Nitroquinoxaline (Intermediate 481)
General Procedure 108: 5-Nitroquinoxaline (Intermediate 481)Oxaldehyde (40% in water; 1.43 ml, 31.3 mmol) was added to a solution of 3-nitro-o-phenylene-diamine (600 mg, 3.9 mmol) in EtOH (15 ml) and the mixture was heated at 85° C. for 2 h. The solvent was removed in vacuo, the residue was diluted in water and the aqueous phase was extracted with DCM. The organic phase was dried (Na2SO4) and concentrated in vacuo. The crude residue was purified by column chromatography with DCM/MeOH (99:1) as the eluent to give the title compound (666 mg, 97%).MW: 175.15HPLCMS: (Method C): [m/z]: 176
References:
US2012/214803,2012,A1 Location in patent:Page/Page column 174
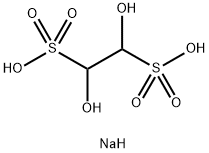
517-21-5
95 suppliers
$17.00/25g

3694-52-8
165 suppliers
$15.00/250mg

18514-76-6
47 suppliers
inquiry
91-19-0
217 suppliers
$20.00/25g

18514-76-6
47 suppliers
inquiry

6639-87-8
110 suppliers
$15.00/100mg

91-19-0
217 suppliers
$20.00/25g
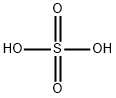
7664-93-9
0 suppliers
$23.30/1090731000
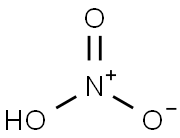
7697-37-2
0 suppliers
$13.00/25g

18514-76-6
47 suppliers
inquiry

100383-24-2
0 suppliers
inquiry