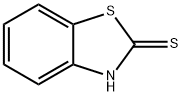
5-Methyl-2-mercaptobenzothiazole synthesis
- Product Name:5-Methyl-2-mercaptobenzothiazole
- CAS Number:21303-50-4
- Molecular formula:C8H7NS2
- Molecular Weight:181.28
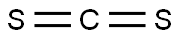
75-15-0

95-81-8

21303-50-4
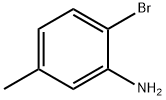
Synthesis of 5-methyl-2-mercaptobenzothiazole (10): 2-chloro-5-methylaniline (9, 1.00 g, 7.06 mmol) and potassium carbonate (K2CO3, 2.80 g, 28.2 mmol) were suspended in N,N-dimethylformamide (DMF, 20 mL) under protection of nitrogen. Subsequently, carbon disulfide (CS2, 0.50 mL, 8.50 mmol) was added to the suspension. The reaction mixture was heated and reacted at 150 °C for 16 hours. Upon completion of the reaction, it was cooled to room temperature, the reaction mixture was diluted with deionized water (30 mL) and acidified with 1 N hydrochloric acid (HCl) to a pH of about 1. The resulting precipitate was collected by filtration, washed several times with deionized water, and finally dried under vacuum in the presence of phosphorus pentoxide (P2O5) to afford the target product 5-methyl-2-mercaptobenzothiazole (10, 0.35 g, Yield 27%).
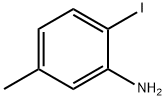
13194-69-9
115 suppliers
$5.00/250mg
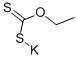
140-89-6
309 suppliers
$12.00/5g

21303-50-4
38 suppliers
inquiry
Yield:21303-50-4 73%
Reaction Conditions:
with iron(III) trifluoride;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in N,N-dimethyl-formamide at 110; for 8 h;Inert atmosphere;Sealed tube;
Steps:
2. General experimental procedure
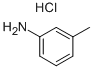
General procedure: A 25 mL reaction tube was charged with 2-haloaniline 1 (0.6 mmol), potassium o-ethyldithiocarbonate 2 (1.8 mmol), FeF3 (0.06 mmol), 2,2’-bis(diphenylphosphino)-1,1’-binaphthyl (0.03 mmol) and DMF (4 mL). The reaction vessel was flushed with argon for three times and sealed. Then the mixture was stirred electromagnetically in an oil bath at 110 for 3 - 21 hours. The reaction process was monitored by TLC on silica gel. After the reaction was completed, the reaction mixture was cooled to room temperature, then 4 mL HCl (3mol/L) was added and stirred for 30 minutes. Then the reaction mixture solution was extracted by ethyl acetate (3*20 mL). Subsequently, the combined organic solution were dried by anhydrous sodium sulfate and the target product was purified by silica gel colum chromatography (eluent: petroleum ether / ethylacetate) to give the corresponding pure product 3.
References:
Gao, Min;Lou, Chunqing;Zhu, Ning;Qin, Weijing;Suo, Quanling;Han, Limin;Hong, Hailong [Synthetic Communications,2015,vol. 45,# 20,p. 2378 - 2385] Location in patent:supporting information

108-44-1
390 suppliers
$9.00/1g

21303-50-4
38 suppliers
inquiry