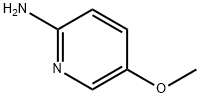
5-METHOXY-PYRIDIN-2-YLAMINE synthesis
- Product Name:5-METHOXY-PYRIDIN-2-YLAMINE
- CAS Number:10167-97-2
- Molecular formula:C6H8N2O
- Molecular Weight:124.14

76066-07-4

10167-97-2
General procedure for the synthesis of 2-amino-5-methoxypyridine from 2-bromo-3-methoxy-6-nitropyridine: 2-bromo-3-methoxy-6-nitropyridine (1.20 g, 5.15 mmol), hydrazine hydrate (6 mL), Pd/C (10%, 400 mg), and ethanol (40 mL) were mixed and heated to reflux for 45 minutes. Upon completion of the reaction, the mixture was allowed to cool and filtered through Celite. The filtrate was concentrated, water (20 mL) and saturated ammonia (10 mL) were added and extracted with chloroform (2 x 50 mL). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated to give 2-amino-5-methoxypyridine as a colorless solid with a low melting point. Yield: 615 mg (96%).1H NMR (DMSO-d6, 400MHz) δ 7.64 (dd, 1H), 7.10 (dd, 1H), 6.42 (dd, 1H), 5.43 (br.s, 2H), 3.68 (s, 3H).
638352-78-0
22 suppliers
$400.00/1g

10167-97-2
221 suppliers
$18.00/1g
Yield:10167-97-2 100%
Reaction Conditions:
with hydroxylamine hydrochloride;triethylamine in ethanol;water; for 20 h;Heating / reflux;
Steps:
20.C
A mixture of 118 (1.0 equiv., 257 mmol, 52.0 g), hydroxylamine hydrochloride(6.5 equiv., 1671 mmol, 69.5 g), triethylamine (2.0 equiv., 514 mmol, 52.0 g), ethanol(400 ml) and water (200 ml) was refiuxed for 20 h. The solution was cooled and
References:
WO2007/113290,2007,A1 Location in patent:Page/Page column 40-42

76066-07-4
98 suppliers
$44.00/250mg

10167-97-2
221 suppliers
$18.00/1g
1072-97-5
705 suppliers
$9.00/1g
124-41-4
727 suppliers
$12.00/25g

10167-97-2
221 suppliers
$18.00/1g
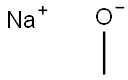
124-41-4
727 suppliers
$12.00/25g

21717-96-4
430 suppliers
$10.00/1g

10167-97-2
221 suppliers
$18.00/1g

20511-12-0
467 suppliers
$6.00/1g
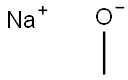
124-41-4
727 suppliers
$12.00/25g

10167-97-2
221 suppliers
$18.00/1g