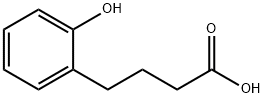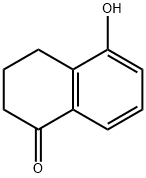
5-Hydroxy-1-tetralone synthesis
- Product Name:5-Hydroxy-1-tetralone
- CAS Number:28315-93-7
- Molecular formula:C10H10O2
- Molecular Weight:162.19
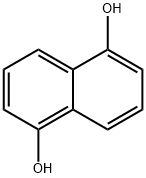
83-56-7

28315-93-7
The general procedure for the synthesis of 5-hydroxy-1-tetralone from 1,5-dihydroxynaphthalene is as follows: Example 1-1 Method A Preparation of [5-(biphenyl-4-sulfonylamino)-5,6,7,8-tetrahydronaphthalen-1-yloxy]-acetic acid; Synthesis of 5-hydroxy-3,4-dihydro-2H-naphthalen-1-one To a mixed solution of isopropanol (150 mL) and aqueous sodium hydroxide (40 mL) containing 1,5-dihydroxynaphthalene (25.0 g, 156 mmol) was added 10% palladium carbon catalyst (3.9 g) at room temperature. The reaction mixture was transferred to a Parr autoclave (Parr Instrument Company) and reacted at 80 °C for 20 h under 100 psi hydrogen pressure. Upon completion of the reaction, the mixture was cooled to room temperature, filtered through a diatomaceous earth pad (diatomaceous earth filter from World Minerals Inc.) and the filter cake was washed with isopropanol (200 mL). The combined filtrates were treated with activated carbon at 50 °C for 1 h and subsequently filtered through a Celite pad (diatomaceous earth filter). Isopropanol was removed by distillation under reduced pressure and the pH was adjusted to about 2 by slowly adding concentrated hydrochloric acid to the remaining solution, at which point a solid precipitated. The solid product was collected, washed with water (100 mL x 2), and then dried under high vacuum at 50 °C to afford 5-hydroxy-3,4-dihydro-2H-naphthalen-1-one (15.0 g, 60% yield) as a dark brown solid, which can be used in subsequent steps without further purification. Mass spectrometry analysis: calculated value (C10H10O2) 162, measured value (M+H)+ 163.

83-56-7
473 suppliers
$9.00/25g

28315-93-7
295 suppliers
$7.00/250mg
Yield:28315-93-7 83.3%
Reaction Conditions:
with palladium on activated charcoal;ammonium formate in water;butan-1-ol at 120; for 3 h;Further stages;Temperature;Solvent;Reagent/catalyst;
Steps:
2 Example 2
Mixing 32 g of 1,5-dihydroxynaphthalene, 8 g of water, 152 g of n-butanol, 3.2 g of palladium on carbon and 12.6 g of ammonium formate,The reaction was heated to 120 ° C under normal pressure for 3 h, and 12 g of sodium hydroxide was added to the obtained system.Stir and heat filtration, and adjust the pH of the obtained filtrate to 3 with industrial hydrochloric acid having a mass concentration of 31%.After the solid was precipitated, it was suction filtered, and the obtained cake was recrystallized from methanol and water (volume ratio of methanol to water: 4:1).Drying in vacuo gave 27.0 g of 5-hydroxy-1-tetralone.The liquid phase purity is greater than 99% and the yield is 83.3%.
References:
Hangzhou Normal University;Hangzhou Lupu Biological Technology Co., Ltd.;Xu Weiming;Zhang Pengfei;Feng Dongxiang;Tao Lianzhi CN109160882, 2019, A Location in patent:Paragraph 0041-0043-0044; 0048-0049; 0051-0052; 0054-0055
33892-75-0
164 suppliers
$8.00/1g

28315-93-7
295 suppliers
$7.00/250mg
40771-26-4
59 suppliers
$85.00/100mg

28315-93-7
295 suppliers
$7.00/250mg

91028-13-6
1 suppliers
inquiry

28315-93-7
295 suppliers
$7.00/250mg
![4,5-Dihydrobenzo[b]oxepin-2(3H)-one](/CAS/20180703/GIF/3041-17-6.gif)