
5-FLUORO-2,3-DIHYDRO-(1H)-INDOLE synthesis
- Product Name:5-FLUORO-2,3-DIHYDRO-(1H)-INDOLE
- CAS Number:94805-51-3
- Molecular formula:C6H4N2O
- Molecular Weight:120.11
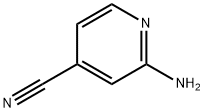
42182-27-4

94805-51-3
General procedure for the synthesis of 2-oxo-1,2-dihydropyridine-4-carbonitrile from 2-amino-4-cyanopyridine: Sodium nitrite (NaNO2, 0.99 g, 14.3 mmol) was added in batches to a well-stirred 2-amino-4-cyanopyridine (0.96 g, 8.1 mmol) dissolved in concentrated sulfuric acid (H2SO4, 1.2 mL) and water (11 mL) in a premixed solution. During the reaction, the temperature of the reaction mixture was maintained between 0-5 °C and strictly controlled in the range of 5 ± 5 °C. With the release of nitrogen (N2), the originally clarified solution gradually became turbid. The mixture was slowly warmed to room temperature with stirring, followed by reflux heating in a water bath for 30 min and finally cooled to room temperature. The solid product formed was collected by filtration, washed with water (H2O) and dried under vacuum to afford the target product 2-oxo-1,2-dihydropyridine-4-carbonitrile (0.9 g, 92% yield) as a colorless solid. The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 8.21 (d, 1H, J = 2.4 Hz), 7.61 (dd, 1H, J = 2.4, 9.6 Hz), 6.38 (d, 1H, J = 9.6 Hz), 3.3 (broad peak, 1H + 2H2O).

42182-27-4
250 suppliers
$10.00/1g

94805-51-3
81 suppliers
$13.00/100mg
Yield: 92%
Reaction Conditions:
with sulfuric acid;sodium nitrite in water at 0; for 0.5 h;Reflux;
Steps:
32.C Step C: 2-Hydroxy-4-cyanopyridine:
NaN02 (0.99 g, 14.3 mmol) was added in small portions to a well stirred solution of the product of Step B (0.96 g, 8.1 mmol) in a premixed solution of concentrated H2S04 (1 .2 ml) and H20 (1 1 .5 ml) while the temperature of the reaction mixture was kept at ) 0 - 5°C. The clear solution became heterogenous with evolution of N2. The mixture was allowed to warm up to room temperature with stirring, than heated on the water bath (reflux) for 30 min and cooled to room temperature. The solid formed was filtered off, washed with H20 and dried in vacuo to give the title product (0.9 g, 92%) as colourless solid. 1 H NMR (DMSO-d6) 8.21 (d, 1 H, J = 2.4 Hz), 7.61 (dd, 1 H, 2.4, 9.6 Hz), 6.38 (d, 1 H, J = 9.6 Hz), 3.3 (broad s, 1 H + 2H20).
References:
AKAAL PHARMA PTY LTD;GROBELNY, Damian W;GILL, Gurmit S WO2014/63199, 2014, A1 Location in patent:Page/Page column 59
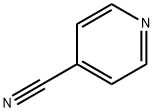
100-48-1
514 suppliers
$8.00/25g

94805-51-3
81 suppliers
$13.00/100mg
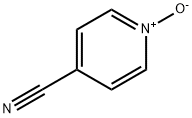
14906-59-3
238 suppliers
$27.00/5g

94805-51-3
81 suppliers
$13.00/100mg

552331-00-7
126 suppliers
$45.00/50mg

94805-51-3
81 suppliers
$13.00/100mg
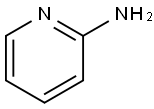
504-29-0
556 suppliers
$5.00/5 g

94805-51-3
81 suppliers
$13.00/100mg