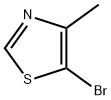
5-bromo-4-methylthiazole synthesis
- Product Name:5-bromo-4-methylthiazole
- CAS Number:111600-83-0
- Molecular formula:C4H4BrNS
- Molecular Weight:178.05
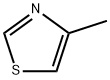
693-95-8

111600-83-0
General procedure for the synthesis of 4-methyl-5-bromothiazole from 4-methylthiazole: 4-methylthiazole (200 μL, 2.2 mmol) was mixed with bromine (112 μL, 2.2 mmol) in acetic acid (2 mL), and the reaction system needed to be protected from light, and the reaction was stirred for 16 hours at room temperature. Upon completion of the reaction, the reaction mixture was washed with 10% aqueous sodium carbonate solution and subsequently extracted with ethyl acetate. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford the target product 4-methyl-5-bromothiazole (0.085 g, 22% yield). Its nuclear magnetic resonance hydrogen spectrum (1H NMR, 400 MHz, CDCl3) data were as follows: δ 2.45 (3H, s), 8.69 (1H, s).

693-95-8
332 suppliers
$6.00/5g

111600-83-0
99 suppliers
$19.00/100mg
Yield: 37%
Reaction Conditions:
with bromine;acetic acid at 0 - 20;
Steps:
8
Preparation 85 -Bromo-4-methylthiazoleAdd bromine (9.27 mL, 182 mmol) to a solution of 4-methylthiazole (15.0 g, 152 mmol) in acetic acid (30 mL) at 0 0C. Slowly warm the reaction mixture to room temperature and stir overnight. Dilute with dichloromethane and wash with 1 N NaOH and brine. Dry the organic layer over sodium sulfate, filter, and concentrate under vacuum. Purify the crude product by silica gel column chromatography, elueting with hexanes/ethyl acetate (5/1) to obtain the title compound (9.94 g, 37%). 1H NMR (400 MHz, CDCl3): δ 8.69 (s, IH), 2.43 (s, 3H).
References:
ELI LILLY AND COMPANY WO2008/36579, 2008, A1 Location in patent:Page/Page column 26
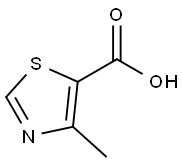
20485-41-0
217 suppliers
$6.00/1g

111600-83-0
99 suppliers
$19.00/100mg