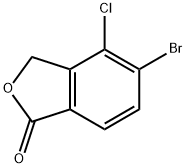
5-bromo-4-chloroisobenzofuran-1(3H)-one synthesis
- Product Name:5-bromo-4-chloroisobenzofuran-1(3H)-one
- CAS Number:1374574-18-1
- Molecular formula:C8H4BrClO2
- Molecular Weight:247.47
201230-82-2
1 suppliers
inquiry
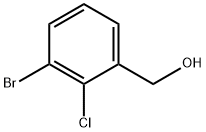
1261524-75-7
54 suppliers
$60.00/100mg

1374574-18-1
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (3-bromo-2-chloro-phenyl)methanolwith trifluoroacetic acid;thallium(III) trifluoroacetate at 20; for 16 h;
Stage #2: carbon monoxidewith magnesium oxide;lithium chloride;palladium dichloride in ethanol at 20; for 2 h;
Steps:
36.D Step D: 5-bromo-4-chloro-2-benzofuran-l(3H)-one
Step D: 5-bromo-4-chloro-2-benzofuran-l(3H)-one: To a flask charged with (3-bromo-2- chlorophenyl)methanol (1.1 g, 4.8 mmol) and a stir bar was added thallium trifluoroacetate (2.9 g, 5.3 mmol) and TFA (6 mL). The mixture was allowed to stir at RT for 16 hours. The volatiles were removed under reduced pressure. The residue was pumped under high vacuum for 15 minutes before palladium (II) chloride (0.085 g, 0.48 mmol), magnesium oxide (0.39 g, 9.6 mmol), lithium chloride (0.20 g, 4.8 mmol), and ethanol (30 mL) were added. The mixture was stirred under an atmosphere of carbon mono-oxide until the reaction turned black. The reaction was diluted with DCM. The suspension was filtered through a pad of celite to remove the solids. The filtrate was adsorbed onto silica gel, and purified by MPLC to afford the title compound.
References:
WO2013/62892,2013,A1 Location in patent:Page/Page column 52
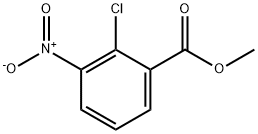
53553-14-3
84 suppliers
$14.00/100mg

1374574-18-1
2 suppliers
inquiry

120100-15-4
42 suppliers
$105.00/250mg

1374574-18-1
2 suppliers
inquiry
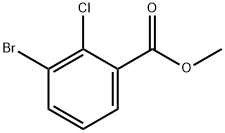
871224-19-0
58 suppliers
$89.00/250mg

1374574-18-1
2 suppliers
inquiry