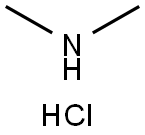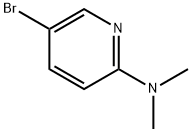
5-Bromo-2-(dimethylamino)pyridine synthesis
- Product Name:5-Bromo-2-(dimethylamino)pyridine
- CAS Number:26163-07-5
- Molecular formula:C7H9BrN2
- Molecular Weight:201.06
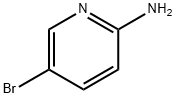
1072-97-5

74-88-4

26163-07-5
Step 64a: Synthesis of 5-bromo-N,N-dimethylpyridin-2-amine (Compound 0601-114) To a solution of 5-bromopyridin-2-amine (1.0 g, 5.8 mmol) in tetrahydrofuran (THF, 25 mL) was added sodium hydride (NaH, 60% dispersed in mineral oil, 0.92 g, 23.1 mmol) at 0°C and the reaction mixture was stirred for 10 minutes. Subsequently, iodomethane (CH3I, 1 mL, 16 mmol) was added and the reaction continued to be stirred for 2 hours. Upon completion of the reaction, the reaction was quenched by the addition of water (30 mL) and extracted with ethyl acetate (3 x 30 mL). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 10% v/v) to give the title compound (1.1 g, 94% yield) as a white solid. LCMS: 203 [M+2]+. 1H NMR (400 MHz, DMSO-d6) δ 2.99 (s, 6H), 6.61 (d, J = 9.6 Hz, 1H), 7.62 (dd, J = 9.2, 2.8 Hz, 1H), 8.12 (d, J = 2.4 Hz, 1H).
Yield:26163-07-5 512 mg
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 90; for 12 h;
Steps:
38.1 Step 1: Preparation of 5-bromo-N,N-lutidine-2-amine (compound 36b)
Compound 36a (650 mg, 3.38 mmol), dimethylamine hydrochloride (550.86 mg, 6.76 mmol) and potassium carbonate (1.4 g, 10.13 mmol) were added to DMF (10.0 mL) and reacted at 90°C for 12 hours.After the reaction, 200 mL of water was added, extracted with EA, the organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and separated and purified by silica gel column chromatography (DCM/MeOH=10/1) to obtain compound 36b (512 mg).
References:
WO2022/89219,2022,A1 Location in patent:Page/Page column 46

766-11-0
458 suppliers
$6.00/5g
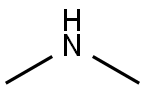
124-40-3
544 suppliers
$18.00/100ml

26163-07-5
114 suppliers
$6.00/1g
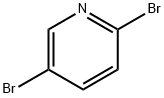
624-28-2
718 suppliers
$5.00/5g

124-40-3
544 suppliers
$18.00/100ml

26163-07-5
114 suppliers
$6.00/1g