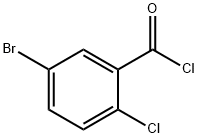
5-BROMO-2-CHLORO-BENZOYL CHLORIDE synthesis
- Product Name:5-BROMO-2-CHLORO-BENZOYL CHLORIDE
- CAS Number:21900-52-7
- Molecular formula:C7H3BrCl2O
- Molecular Weight:253.91
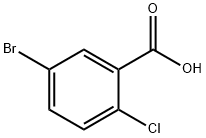
21739-92-4

21900-52-7
Step 1. 5-Bromo-2-chlorobenzoic acid (100 g, 0.43 mol) was dissolved in 500 mL of dichloromethane and 1 g of pyridine was added as a catalyst. Thionyl chloride (60.5 g, 0.51 mol) was added slowly and dropwise at room temperature, followed by heating the reaction mixture to 40 °C and refluxing for 3.5 hours. The reaction progress was monitored by thin layer chromatography (TLC). Upon completion of the reaction, dichloromethane was removed by distillation under reduced pressure to afford 104 g of 5-bromo-2-chlorobenzoyl chloride (yield: 97%), which can be used directly in the next step of the reaction without further purification.
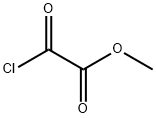
5781-53-3
240 suppliers
$8.00/10g
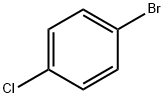
106-39-8
462 suppliers
$14.00/5g

21900-52-7
84 suppliers
$12.00/250mg
Yield: 98.9%
Reaction Conditions:
Stage #1:monomethyl oxalyl chloride with copper sulfate supported molecular sieve in 1,1,1-trichloroethane at 125; under 4560.31 Torr; for 1.5 h;Inert atmosphere;
Stage #2:bromochlorobenzene in chloroform;1,1,1-trichloroethane at 165; under 6080.41 Torr; for 14 h;Inert atmosphere;Temperature;Pressure;
Steps:
1-8 A method for synthesizing 5-bromo-2-chlorobenzoyl chloride, comprising the steps of:
Mix oxalyl chloride monomethyl ester with 1,1,1-trichloroethane, add catalyst, stir and pass argon gas, control the pressure to 6 atmospheres, control the temperature to 125 ° C, maintain 1.5 h, Then, a solution of 4-bromochlorobenzene and chloroform was added dropwise, and after the addition, the temperature of the system was raised to 165 ° C, and the pressure was raised to 8 atm to continue the reaction for 14 hours.The catalyst is obtained by grinding the 4A molecular sieve, after 350 mesh, immersing in a 12% mass fraction of copper sulfate aqueous solution for 4 days, filtering, and baking at 400 ° C to obtain the weight ratio of molecular sieve to copper sulfate aqueous solution; It is 1:7.2.The molar ratio of 4-bromochlorobenzene to oxalyl chloride monomethyl ester is 1:1.12; the ratio of oxalyl chloride monomethyl ester to 1,1,1-trichloroethane is lg: 11ml; 4-bromochlorobenzene and chloroform The dosage ratio is lg: 8 ml; the weight ratio of 4-bromochlorobenzene to the catalyst is 1:1.15.2) After cooling, the solid was removed by filtration, and the filtrate was added to a volume of 1 to 2 volumes of toluene, washed with water to pH = 7. The mixture was dried over anhydrous sodium sulfate and evaporated to give a product. The molar yield was 98.9%, and the GC purity was 99.1%.
References:
Shenzhen The Second People Hospital;Tan Hui;Li Weiping;Huang Xianjian;Tang Aifa;Liu Wenlan;Chen Lei CN109456177, 2019, A Location in patent:Paragraph 0031-0059

21739-92-4
683 suppliers
$8.00/10g

21900-52-7
84 suppliers
$12.00/250mg
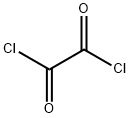
79-37-8
487 suppliers
$17.67/10gm:

21739-92-4
683 suppliers
$8.00/10g

21900-52-7
84 suppliers
$12.00/250mg
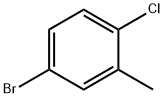
54932-72-8
216 suppliers
$6.00/1g

21900-52-7
84 suppliers
$12.00/250mg
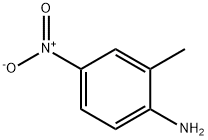
99-52-5
282 suppliers
$5.00/5 g

21900-52-7
84 suppliers
$12.00/250mg