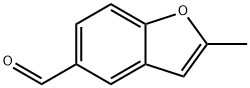
5-Benzofurancarboxaldehyde, 2-methyl- (9CI) synthesis
- Product Name:5-Benzofurancarboxaldehyde, 2-methyl- (9CI)
- CAS Number:204584-97-4
- Molecular formula:C10H8O2
- Molecular Weight:160.17
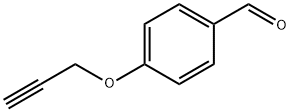
5651-86-5
58 suppliers
$14.00/1G

204584-97-4
1 suppliers
inquiry
Yield:204584-97-4 24%
Reaction Conditions:
at 220; for 4 h;
Steps:
2 Step 2: Preparation of 2-methyl-benzofuran-5-carbaldehyde (D):
To compound C (3 g, 18.75 mmol, 1 eq) was added PEG-300 (30 mL) and the resulting mixture was heated to 220 °C for 4h. The reaction mixture was cooled to RT, diluted with ice-water (70 ml) and the organic components were extracted with ethyl acetate (0200) (200 ml). The ethyl acetate layer was concentrated in vacuo and the crude material was purified by flash chromatography (Combiflash) using 100-200 mesh silica gel eluting with (0201) 20% ethyl acetate/ hexane to obtain the compound D (700 mg, 24%) as white solid. (0202) [0195] FontWeight="Bold" FontSize="10" H NMR (400 MHz, DMSO-cfe) δ 10.02 (s, 1 H), 8.12 (s, 1 H), 7.80-7.78 (m, 1 (0203) H), 7.69-7.67 (m, 1 H), 6.76 (s, 1 H), 2.48 (s, 3 H); (0204) [0196] LCMS: m/z = 161.2 [M+H], RT = 3.22 minutes; (Program Rl, Column Y).
References:
WO2018/64135,2018,A1 Location in patent:Paragraph 0193-0196
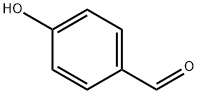
123-08-0
952 suppliers
$5.00/10g

204584-97-4
1 suppliers
inquiry