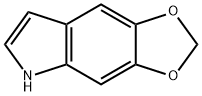
5,6-Methylenedioxyindole synthesis
- Product Name:5,6-Methylenedioxyindole
- CAS Number:267-48-1
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
![Phosphonium, [2-(1,1-dimethylethoxy)-2-oxoethyl]triphenyl-, inner salt](/CAS/20211123/GIF/86302-43-4.gif)
86302-43-4

712-97-0

267-48-1
The general procedure for the synthesis of 5,6-methylenedioxyindole from [2-(1,1-dimethylethoxy)-2-oxoethyl]triphenylphosphonium inner salt and 6-nitropiperonyl aldehyde was as follows: In a 50 mL round-bottomed flask equipped with a magnetic stirrer, 6-nitropiperonyl aldehyde (2 mmol), [2-(1,1-dimethylethoxy)-2-oxoethyl]triphenylphosphonium inner salt (2.2 mmol), triphenylphosphine (4.6 mmol), and diphenyl ether (10 mL) were added in sequence. mmol), triphenylphosphine (4.6 mmol) and diphenyl ether (10 mL). The reaction mixture was heated to 260 °C and kept for 1 hour. Upon completion of the reaction, the mixture was cooled to room temperature and the crude product was directly sampled onto a silica gel column. Separation was carried out by column chromatography using a gradient elution system of petroleum ether to 3:1 (v/v) petroleum ether/ethyl acetate to purify the target product 5,6-methylenedioxyindole.
![5H-1,3-Dioxolo[4,5-f]indole, 5-(phenylsulfonyl)-](/CAS/20210305/GIF/100587-74-4.gif)
100587-74-4
1 suppliers
inquiry

267-48-1
87 suppliers
$17.00/100mg
Yield:267-48-1 93%
Reaction Conditions:
with sodium hydroxide in N,N-dimethyl-formamide at 60; for 2 h;Large scale;
Steps:
4 Fourth step reaction formula
16.3kg DMF, 9.5kg A4 and 41kg 20% sodium hydroxide solution were added to the kettle. The temperature was raised to 60°C, and the mixture was stirred for 2 hours. The A4 detected by HPLC was less than 0.5%. In the kettle, the solvent was removed under reduced pressure, most of the solvent was distilled off, extracted with 15kg×2MTBE, combined the organic phases, and washed with 10kg of water. Filter with 2kg of silica gel, spin dry, and beat with EA twice. After suction filtration, 2.3kg of 5,6-methylenedioxyindole A5 is obtained, which is a white solid. The yield is 93%, and the HPLC purity is 99.5%
References:
CN112574226, 2021, A Location in patent:Paragraph 0036-0038
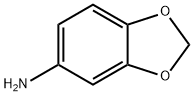
14268-66-7
273 suppliers
$20.00/5 g
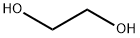
107-21-1
1368 suppliers
$10.00/25g

267-48-1
87 suppliers
$17.00/100mg

63375-82-6
1 suppliers
inquiry

267-48-1
87 suppliers
$17.00/100mg

712-97-0
119 suppliers
$16.00/1 g

267-48-1
87 suppliers
$17.00/100mg