
(4-Hydroxymethylthiazol-2-yl)carbamic acid tert-butyl ester synthesis
- Product Name:(4-Hydroxymethylthiazol-2-yl)carbamic acid tert-butyl ester
- CAS Number:494769-44-7
- Molecular formula:C9H14N2O3S
- Molecular Weight:230.28
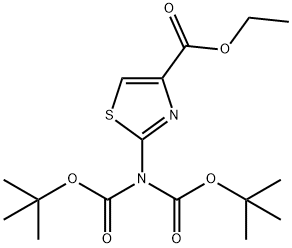
930303-58-5

494769-44-7
Step 2: Synthesis of tert-butyl (4-hydroxymethylthiazol-2-yl)carbamate Ethyl 2-(bis(tert-butoxycarbonyl)amino)thiazole-4-carboxylate (8.0 g, 21.5 mmol) was dissolved in 160 mL of tetrahydrofuran (THF), and cooled to -15 °C. Under stirring, lithium aluminum hydride (1 M in THF, 23.5 mL, 23.6 mmol) was added slowly and dropwise, keeping the reaction temperature at -15 °C. The reaction mixture was continued to be stirred at -15 °C for 1 hour. Upon completion of the reaction, the reaction was quenched by careful addition of water and subsequently washed with saturated aqueous D-tartaric acid potassium solution. The mixture was extracted three times with ethyl acetate and the organic layers were combined. The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by fast column chromatography on silica gel with ethyl acetate/heptane as eluent (5:95→50:50 gradient). The target product (4-hydroxymethylthiazol-2-yl)carbamic acid tert-butyl ester was obtained as a light yellow solid (3.2 g, 77% yield), mass spectrum (MS): m/e = 231.2 ([M+H]+).

930303-58-5
3 suppliers
inquiry

494769-44-7
95 suppliers
$10.00/250mg
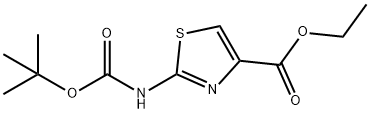
189512-01-4
45 suppliers
$60.00/250mg

494769-44-7
95 suppliers
$10.00/250mg

850429-62-8
76 suppliers
$30.00/1 g

494769-44-7
95 suppliers
$10.00/250mg
