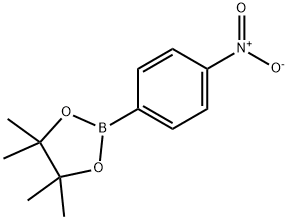
4-Nitrophenylboronic acid pinacol ester synthesis
- Product Name:4-Nitrophenylboronic acid pinacol ester
- CAS Number:171364-83-3
- Molecular formula:C12H16BNO4
- Molecular Weight:249.07
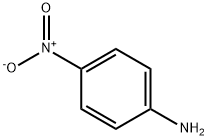
100-01-6
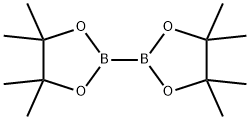
73183-34-3

171364-83-3
GENERAL PROCEDURE: To a solution of 4-nitroaniline (0.5 mmol, 1.0 eq.) in methanol (1.0 mL) was added hydrochloric acid (0.5 mL, 1.5 mmol, 3.0 eq.) followed by water (0.5 mL). The mixture was stirred for 2 minutes and then sodium nitrite solution (0.25 mL) was added. Sodium nitrite solution was prepared by dissolving 35 mg of sodium nitrite in water (0.25 mL). The reaction mixture was stirred at 0-5 °C for 30 min, followed by the addition of a methanol (1.0 mL) solution of pinacol ester of bisboronic acid (2,381 mg, 1.5 mmol, 3.0 equiv). Stirring was continued for 60 minutes. Upon completion of the reaction, water (10 mL) was added to the mixture, which was then extracted with dichloromethane (50 mL, 3 times). The organic layers were combined and washed with saturated sodium bicarbonate solution followed by drying with anhydrous sodium sulfate. After drying, the solvent was evaporated and the crude product was purified by fast chromatography.
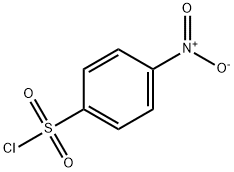
98-74-8
173 suppliers
$10.00/5g

73183-34-3
589 suppliers
$6.00/5g

171364-83-3
183 suppliers
$7.00/250mg
Yield: 78%
Reaction Conditions:
with dipotassium hydrogenphosphate in acetonitrile at 20; for 24 h;UV-irradiation;
Steps:
4 Synthesis of 4-nitrophenylboronic acid ester
To a 25 mL reaction tube was added 2 mL of acetonitrile, 4-methylbenzenesulfonyl chloride (66.3 mg, 0.3 mmol), boronic acid pinacol ester (114.3 mg, 0.45 mmol), dipotassium hydrogen phosphate (104.4 mg, 2.0 eq.) , And the reaction tube was irradiated with an ultraviolet lamp, and the reaction was carried out by stirring at room temperature for 24 hours.After the reaction was completed, most of the solvent was distilled off under reduced pressure, and the remaining mixture was purified by column chromatography on petroleum ether / ethyl acetate (10: 1) as eluent to obtain the desired product as a yellow solid , 58.3 mg, yield 78%.
References:
Lishui University;YAN, GUO BING;YU, JIAN CN105859761, 2016, A Location in patent:Paragraph 0029-0031

100-01-6
66 suppliers
$11.19/25G

73183-34-3
589 suppliers
$6.00/5g

171364-83-3
183 suppliers
$7.00/250mg
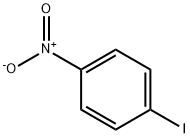
636-98-6
34 suppliers
$17.00/5g

73183-34-3
589 suppliers
$6.00/5g

171364-83-3
183 suppliers
$7.00/250mg
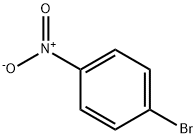
586-78-7
18 suppliers
$14.00/5g

73183-34-3
589 suppliers
$6.00/5g

171364-83-3
183 suppliers
$7.00/250mg
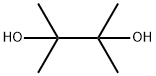
76-09-5
395 suppliers
$8.19/250mg

22092-92-8
3 suppliers
inquiry
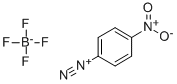
456-27-9
155 suppliers
$17.89/1g

171364-83-3
183 suppliers
$7.00/250mg