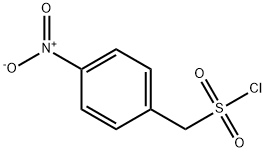
(4-Nitrophenyl)methanesulfonyl chloride synthesis
- Product Name:(4-Nitrophenyl)methanesulfonyl chloride
- CAS Number:4025-75-6
- Molecular formula:C7H6ClNO4S
- Molecular Weight:235.64
64039-36-7

4025-75-6
The general procedure for the synthesis of (4-nitrophenyl)methanesulfonyl chloride from 4-nitrophenylaminosulfate methyl ester hydrochloride is as follows: 1. the alkyl halide (or sulfate) (5 mmol) was heated with thiourea (0.381 g, 5 mmol) in ethanol (5 mL) at reflux for 1 hour. 2. Upon completion of the reaction, the solvent was removed under vacuum and the residue was washed with ether (3 x 5 mL) to afford the corresponding S-alkyl isothiourea salt as a white solid in near quantitative yield. 3. Without further purification, the resulting S-alkylisothiourea salt was transferred to a three-necked round-bottomed flask equipped with a thermometer and a charging funnel and placed in an ice bath. 4. Water (0.45 mL) and acetonitrile (10 mL) were added to the flask to form a vigorously stirred mixture. 5. A solution of tert-butyl hypochlorite (t-BuOCl, 2.86 mL) in acetonitrile (5 mL) was added slowly and dropwise while maintaining an internal temperature of 0-20°C. The solution was then mixed with water (0.45 mL) and acetonitrile (10 mL). 6. After the dropwise addition was completed, the reaction mixture was continued to be stirred for 30 minutes. 7. Upon completion of the reaction, the solvent was removed under vacuum and ether (15 mL) was added to dissolve the residue. 8. The ether layer was washed with water (2 x 10 mL) and dried with anhydrous sodium sulfate (Na2SO4). 9. 9. After drying, the ether solution is concentrated in vacuum to give high purity (4-nitrophenyl)methanesulfonyl chloride. 10. The product can be further purified by recrystallization from a mixed petroleum ether-ethyl acetate solvent.

64039-36-7
24 suppliers
inquiry

4025-75-6
105 suppliers
$6.00/250mg
Yield: 88%
Reaction Conditions:
with tert-butylhypochlorite in water;acetonitrile at 0 - 20;
Steps:
Sulfonyl Chlorides 3; General Procedure
General procedure: Alkyl halide (or sulfate) (5 mmol) and thiourea (0.381 g, 5 mmol) were heated at reflux in EtOH (5 mL) for 1 h. After removal of the solvent in vacuum and washing with Et2O (3 × 5 mL), the corresponding S-alkyl isothiourea salt was obtained as a white solid in almost quantitative yield. Without purification, the product was transferred into a three-necked round-bottom flask equipped with a thermometer and an addition funnel in an ice-bath, followed by addition of water (0.45mL) and MeCN (10 mL). To the resultant vigorously stirred mixture was added dropwise a solution of t-BuOCl (2.86 mL) in MeCN (5 mL), keeping the inner temperature at 0-20 °C. The mixture was then stirred for 30 min. Removal of the solvent under vacuum, addition of Et2O (15 mL), washing with H2O (2 × 10 mL), drying over Na2SO4, and concentration under vacuum gave the desired product in high purity. The product was further purified by recrystalization from petroleum ether-EtOAc.
References:
Qiu, Kui;Wang, Rennan [Synthesis,2015,vol. 47,# 20,art. no. SS-2015-H0078-OP,p. 3186 - 3190]
53992-33-9
2 suppliers
inquiry

4025-75-6
105 suppliers
$6.00/250mg

36639-50-6
57 suppliers
inquiry

4025-75-6
105 suppliers
$6.00/250mg
![Ethanethioic acid, S-[(4-nitrophenyl)methyl] ester](/CAS/20210111/GIF/170640-75-2.gif)
170640-75-2
4 suppliers
inquiry

4025-75-6
105 suppliers
$6.00/250mg
![AMINO[(4-NITROBENZYL)SULFANYL]METHANIMINIUM CHLORIDE](/StructureFile/ChemBookStructure21/GIF/CB6787644.gif)
4357-96-4
17 suppliers
$144.00/250mg

4025-75-6
105 suppliers
$6.00/250mg